Clinical effect and prognosis of sacubitril-valsartan in treating heart failure patients with different ejection fraction
-
摘要:
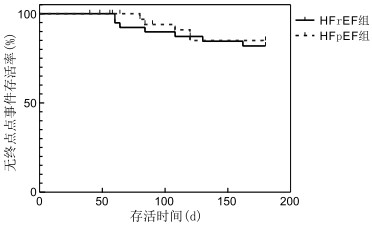
目的 观察沙库巴曲缬沙坦对于不同射血分数慢性心力衰竭(chronic heart failure, CHF)患者的疗效、预后及治疗安全性。 方法 选取2019年1—12月于安徽医科大学第一附属医院心内科住院的CHF患者78例,根据射血分数情况分为射血分数减少(HFrEF)组(共40例)和射血分数保留(HFpEF)组(共38例)。2组患者均接受常规心力衰竭治疗,在此基础上均接受沙库巴曲缬沙坦治疗。比较2组治疗6个月后脑钠肽(BNP)、血钾、肾功能和左房内径(LAD)、左室舒张末内径(LVEDD)及纽约心功能分级(NYHA)变化,并记录治疗的安全性。同时以心血管死亡或心衰再住院为终点事件,采用Kaplan-Meier生存曲线法比较2组患者治疗6个月内累积无终点事件生存率。 结果 HFrEF组治疗6个月后较其治疗前BNP水平下降,LAD、LVEDD减低,NYHA分级改善(均P<0.05);HFpEF组治疗6个月后亦较其治疗前BNP水平下降,LAD、LVEDD减低,NYHA分级改善(均P<0.05);HFrEF组患者BNP水平的下降程度和NYHA分级的改善程度较HFpEF组更佳;HFrEF组和HFpEF组累积无终点事件生存率比较差异无统计学意义(81.9% vs. 85.0%, P=0.694)。2组安全性均较好。 结论 沙库巴曲缬沙坦对于射血分数保留性心衰有一定的疗效,在临床上值得推荐使用,但与射血分数减低性心衰相比疗效稍差。两者6个月累积无终点事件生存率和安全性方面未见明显差异。 Abstract:Objective To observe the clinical effect, prognosis and safety of sacubitril-valsartan in treating chronic heart failure (CHF) patients with different ejection fraction. Methods Seventy-eight patients were obtained from the Department of Cardiology of The First Affiliated Hospital of Anhui Medical University from January 2019 to December 2019. The patients were divided into the HFrEF group and HFpEF group on the basis of ejection fraction: 40 patients in the HFrEF group and 38 patients in the HFpEF group. All patients were treated with sacubitril-valsartan on the basis of routine heart failure treatment. Brain natriuretic peptide (BNP), renal function, serum potassium, left ventricular ejection fraction (LVEF), left atrial diameter (LAD), left ventricular end diastolic diameter (LVEDD) and New York heart function classification (NYHA) of both groups were compared after 6 months of treatment. Treatment safety was also assessed. The end point event was cardiovascular death or hospitalisation for worsening heart failure. The accumulated end-point event-free survival rate in 6 months between the two groups was compared by the Kaplan-Meier survival curve method. Results After 6 months of treatment, the HFrEF group showed significantly decreased BNP, LAD and LVEDD; significantly increased LVEF and significantly improved NYHA classification (all P < 0.05) compared with that before treatment. After 6 months of treatment, the HFpEF group also showed significantly decreased BNP, LAD and LVEDD; significantly increased LVEF and significantly improved NYHA classification (all P < 0.05) compared with that before treatment. However, the degree of decrease in the BNP levels and improvement in NYHA class were better in the HFrEF group than that in the HFpEF group. No difference in cumulative event-free survival was found between the HFrEF and HFpEF groups (81.9% vs. 85.0%, P=0.694). Safety was good in both groups. Conclusion Sacubitril-valsartan has a certain effect on heart failure with preserved ejection fraction, which shows potential clinical application, but it is slightly less effective on heart failure with reduced ejection fraction. There is no significant difference in prognosis and safety between the two groups. -
Key words:
- Sacubitril-valsartan /
- Heart failure /
- Ejection fraction
-
表 1 2组慢性心力衰竭患者基线资料比较[例(%)]
组别 例数 年龄(x ±s,岁) 女性 NYHA分级 LAD[M(P25,P75),cm] LVEDD(x ±s,cm) 高血压 Ⅰ级和Ⅱ级 Ⅲ级 Ⅳ级 HFrEF组 40 61.5±17.5 15(37.5) 5(12.5) 22(55.0) 13(32.5) 4.6(4.2, 5.2) 6.53±0.70 16(40.0) HFpEF组 38 71.0±9.5 18(47.4) 13(34.2)c 19(50.0) 6(15.8) 4.6(4.5, 5.1) 5.39±0.84 26(68.4) 统计量 -2.181a 0.778b -2.444d -0.065d 5.318a 6.334b P值 0.014 0.378 0.015 0.948 <0.001 0.012 组别 例数 冠心病 糖尿病 房颤 BNP[M(P25,P75),pg/mL] Scr[M(P25,P75),μmol/L] eGFR[x ±s,mL/(min·1.73m2)] 血钾(x ±s,mmol/L) HFrEF组 40 14(35.0) 8(20.0) 9(22.5) 1 327.9(957.0, 2 672.7) 84.2(74.3, 96.9) 83.0±26.9 4.0±0.5 HFpEF组 38 12(31.6) 10(26.3) 18(47.4) 833.7(607.5, 1 117.0) 67.3(56.1, 88.1) 84.4±18.5 3.9±0.5 统计量 0.103b 0.438b 5.325b -2.652d -2.189d -0.201a 0.905a P值 0.749 0.508 0.021 0.008 0.029 0.842 0.370 注:a为t值,b为χ2值,d为Z值; HFpEF组与HFrEF组比较,cP < 0.05。 表 2 2组慢性心力衰竭患者治疗前后各指标比较[M(P25,P75)]
组别 例数 BNP(pg/mL) LAD(cm) LVEDD(x ±s,cm) NYHA Ⅰ级和Ⅱ级[例(%)] NYHA Ⅲ级[例(%)] 治疗前 治疗后 治疗前 治疗后 治疗前 治疗后 治疗前 治疗后 治疗前 治疗后 HFrEF组 40 1 327.9(905.7, 2 921.6) 165.9(56.9, 545.5)b 4.6(4.2, 5.2) 4.4(4.0, 4.9)b 6.53±0.70 6.34±0.80b 5(12.5) 20(50.0) 22(55.0) 13(32.5) HFpEF组 38 833.7(607.5, 1 117.0) 175.2(80.0, 393.2)b 4.6(4.5, 5.1) 4.5(4.2,5.1)b 5.39±0.84 5.28±0.78b 13(34.2) 19(50.0) 19(50.0) 14(36.8) 统计量 -2.652a -0.297a -0.065a -0.614a 5.318c 4.404c 5.174d < 0.001d 0.195d 0.162d P值 0.008 0.767 0.948 0.539 <0.001 <0.001 0.023 >0.999 0.659 0.687 组别 例数 NYHA Ⅳ级[例(%)] Scr(μmol/L) eGFR[mL/(min·1.73m2)] 血钾(x ±s,mmol/L) 治疗前 治疗后 治疗前 治疗后 治疗前 治疗后 治疗前 治疗后 HFrEF组 40 13(32.5) 7(17.5) 84.2(74.3, 96.9) 83.2(69.1,99.6) 84.5(65.3,97.5) 75.0(60.0, 104.0) 4.0±0.5 4.3±0.8 HFpEF组 38 6(15.8) 5(13.2) 67.3(56.1, 88.1) 82.2(58.8,95.7) 84.0(75.0,99.0) 75.0(61.0, 96.0) 3.9±0.5 4.1±0.5 统计量 2.953d 0.282d -2.189a -0.600a -0.201a -0.431a 0.905c -0.996c P值 0.086 0.595 0.029 0.549 0.842 0.668 0.370 0.319 注:治疗前,2组NYHA分级比较,Z=-2.444,P=0.015;治疗后,2组NYHA分级比较,Z=-0.181,P=0.856。与治疗前比较,bP<0.05;a为Z值;c为t值,d为χ2值。 -
[1] PONIKOWSKI P, VOORS A A, ANKER S D, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology(ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC[J]. Eur J Heart Fail, 2016, 18(8): 891-975. doi: 10.1002/ejhf.592 [2] KHDER Y, SHI V, MCMURRAY J, et al. Sacubitril/Valsartan (LCZ696) in heart failure[J]. Handb Exp Pharmacol, 2017, 243: 133-165. http://www.ncbi.nlm.nih.gov/pubmed/28004291 [3] MCMURRAY J J, PACKER M, DESAI A S, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure[J]. N Engl J Med, 2014, 371(11): 993-1004. doi: 10.1056/NEJMoa1409077 [4] SOLOMON S D, MCMURRAY J J V, ANAND I S, et al. Angiotensin-neprilysin inhibition in heart failure with preserved ejection fraction[J]. N Engl J Med, 2019, 381(17): 1609-1620. doi: 10.1056/NEJMoa1908655 [5] 中华医学会心血管病学分会心力衰竭学组, 中国医师协会心力衰竭专业委员会, 中华心血管病杂志编辑委员会. 中国心力衰竭诊断和治疗指南2018[J]. 中华心血管病杂志, 2018, 46(10): 760-789. doi: 10.3760/cma.j.issn.0253-3758.2018.10.004 [6] 廖玉华, 杨杰孚, 张健, 等. 舒张性心力衰竭诊断和治疗专家共识[J]. 临床心血管病杂志, 2020, 36(1): 1-10. https://www.cnki.com.cn/Article/CJFDTOTAL-LCXB202001001.htm [7] PIESKE B, TSCHÖPE C, DE BOER R A, et al. How to diagnose heart failure with preserved ejection fraction: the HFA-PEFF diagnostic algorithm: a consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC)[J]. Eur J Heart Fail, 2020, 22(3): 391-412. doi: 10.1002/ejhf.1741 [8] IBRAHIM N E, SONG Y, CANNON C P, et al. Heart failure with mid-range ejection fraction: characterization of patients from the PINNACLE Registry (R)[J]. ESC Heart Fail, 2019, 6(4): 784-792. doi: 10.1002/ehf2.12455 [9] GLADDEN J, CHAANINE A H, REDFIELD M M. Heart failure with preserved ejection fraction[J]. Annu Rev Med, 2018, 69: 65-79. doi: 10.1146/annurev-med-041316-090654 [10] HAN Y, AYALASOMAYAJULA S, PAN W, et al. Pharmacokinetics, safety and tolerability of Sacubitril/Valsartan (LCZ696) after single-dose administration in healthy Chinese subjects[J]. Eur J Drug Metab Pharmacokinet, 2017, 42(1): 109-116. doi: 10.1007/s13318-016-0328-3 [11] ESTEBAN-FERNáNDEZ A, DíEZ-VILLANUEVA P, VICENT L, et al. Sacubitril/Valsartan is useful and safe in elderly people with heart failure and reduced ejection fraction. Data from a real-word cohort[J]. Rev Esp Geriatr Gerontol, 2020, 55(2): 65-69. doi: 10.1016/j.regg.2019.10.002 [12] PASCUAL-FIGAL D, WACHTER R, SENNI M, et al. NT-proBNP response to Sacubitril/Valsartan in hospitalized heart failure patients with reduced ejection fraction: TRANSITION study[J]. JACC Heart Fail, 2020, 8(10): 822-833. doi: 10.1016/j.jchf.2020.05.012 [13] CUNNINGHAM J W, VADUGANATHAN M, CLAGGETT B L, et al. Effects of Sacubitril/Valsartan on N-Terminal Pro-B-Type natriuretic peptide in heart failure with preserved ejection fraction[J]. JACC Heart Fail, 2020, 8(5): 372-381. doi: 10.1016/j.jchf.2020.03.002 [14] VADUGANATHAN M, JHUND P S, CLAGGETT B L, et al. A putative placebo analysis of the effects of sacubitril/valsartan in heart failure across the full range of ejection fraction[J]. Eur Heart J, 2020, 41(25): 2356-2362. doi: 10.1093/eurheartj/ehaa184 [15] SHAH K S, XU H L, MATSOUAKA R A, et al. Heart failure with preserved, borderline, and reduced ejection fraction 5-year outcomes[J]. J Am Coll Cardiol, 2017, 70(20): 2476-2486. doi: 10.1016/j.jacc.2017.08.074 [16] HOONG C W, LIM C P, GAO F, et al. Outcomes of heart failure with preserved ejection fraction in a Southeast Asian cohort[J]. J Cardiovasc Med (Hagerstown), 2015, 16(9): 583-590. doi: 10.2459/JCM.0000000000000100 [17] HSIAO F C, WANG C L, CHANG P C, et al. Angiotensin receptor neprilysin inhibitor for patients with heart failure and reduced ejection fraction: real-world experience from Taiwan[J]. J Cardiovasc Pharmacol Ther, 2020, 25(2): 152-157. doi: 10.1177/1074248419872958 [18] TRIPOSKIADIS F, BUTLER J, ABBOUD F M, et al. The continuous heart failure spectrum: moving beyond an ejection fraction classification[J]. Eur Heart J, 2019, 40(26): 2155-2163. doi: 10.1093/eurheartj/ehz158 [19] GE J B. Coding proposal on phenotyping heart failure with preserved ejection fraction: A practical tool for facilitating etiology-oriented therapy[J]. Cardiol J, 2020, 27(1): 97-98. doi: 10.5603/CJ.2020.0023 -





 下载:
下载: