Observation of curative effect and analysis of prognostic factors of S-1 adjuvant chemotherapy after radical concurrent chemoradiotherapy for esophageal squamous cell carcinoma in the elderly
-
摘要:
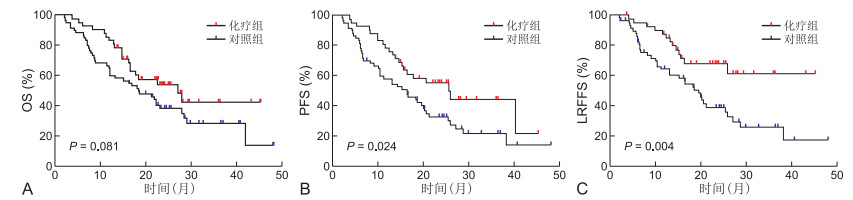
目的 回顾性分析经根治性同步放化疗(CCRT)后替吉奥(S-1)辅助化疗的老年食管鳞癌患者的临床疗效及预后因素。 方法 选取2016年6月1日—2019年6月30日共计102例老年食管鳞癌患者(≥70岁)纳入研究,所有患者既往均于蚌埠医学院第一附属医院放疗科接受过根治性同步放化疗根据是否行替吉奥辅助化疗分为化疗组(42例)和对照组(60例)对T分期、辅助化疗、原发肿瘤长度(GTV长度)等进行Kaplan-Meier统计学分析。 结果 化疗组与对照组2年、3年总生存率(OS)分别为54.1%、42.6%和38.4%、28.5%(P=0.081),2年、3年无进展生存率(PFS)分别为55.4%、44.3%和32.9%、21.8%(P=0.024),2年、3年无局部区域复发生存率(LRFFS)分别为68.2%、61.4%和39.2%、25.9%(P=0.004)COX模型多因素分析结果显示,辅助化疗有着更好的PFS和LRFFS(均P<0.05),临床分期Ⅱ~Ⅲ期相较于Ⅳa期有着更好的OS、PFS及LRFFS,淋巴结状态(N状态)与OS及PFS密切相关,年龄是OS的独立预后因素。 结论 对于老年食管鳞癌患者,经同步放化疗后替吉奥辅助化疗可以提高无进展生存率和无局部区域复发生存率,相关不良反应发生率低且能够耐受。 Abstract:Objective To analyze the clinical efficacy and prognostic factors of the elderly patients with esophageal squamous cell carcinoma who underwent radical concurrent chemoradiotherapy (CCRT) followed by S-1 adjuvant chemotherapy. Methods A total of 102 elderly patients with esophageal squamous cell carcinoma (≥ 70 years old) were enrolled in this study from June 1, 2016 to June 30, 2019. All the patients had received radical concurrent chemoradiotherapy in the Radiotherapy Department of the First Affiliated Hospital of Bengbu Medical College. The patients were divided into chemotherapy group (42 cases) and control group (60 cases) according to whether or not they received S-1 adjuvant chemotherapy. Kaplan-Meier statistical analysis was conducted for T staging, adjuvant chemotherapy and length of primary tumor (GTV length). Results The 2-year and 3-year overall survival rates (OS) in the chemotherapy group and the control group were 54.1%, 42.6% and 38.4%, 28.5% (P=0.081), the 2-year and 3-year progression free survival rates (PFS) were 55.4%, 44.3% and 32.9%, 21.8% (P=0.024), and the 2-year and 3-year local recurrence free survival rates (LRFFS) were 68.2%, 61.4% and 39.2%, 25.9% (P=0.004), respectively. Cox model multivariate analysis showed that adjuvant chemotherapy had better PFS and LRFFS (all P < 0.05). Clinical stage Ⅱ-Ⅲ had better OS, PFS and LRFFS than stage Ⅳ. lymph node status (N status) was closely related to OS and PFS. Age was an independent predictor of OS. Conclusion For elderly patients with esophageal squamous cell carcinoma, after concurrent chemoradiotherapy, S-1 adjuvant chemotherapy can improve PFS and LRFFS, and the incidence of related adverse reactions is low and can be tolerated. -
Key words:
- Esophageal neoplasms /
- Squamous cell carcinoma /
- Adjuvant chemotherapy /
- S-1
-
表 1 2组老年食管鳞癌患者一般临床资料比较
(例) 组别 例数 年龄 性别 吸烟 饮酒 原发肿瘤位置 GTV长度(cm) 基础疾病 PGTV剂量(Gy) T分期 淋巴结状态(N状态) 临床分期 <76岁 ≥76岁 男性 女性 有 无 有 无 颈、上 中、下 <6.5 ≥6.5 有 无 <60 ≥60 2~3 4 N+ N- Ⅱ~Ⅲ Ⅳa 化疗组 42 22 20 29 13 13 29 9 33 16 26 19 23 18 24 6 36 24 18 20 22 22 20 对照组 60 20 40 39 21 17 43 14 46 17 43 22 38 21 39 11 49 31 29 19 41 30 30 χ2值 3.701 0.182 0.082 0.051 1.076 0.755 0.646 0.291 0.298 2.662 0.056 P值 0.054 0.67 0.775 0.821 0.3 0.385 0.422 0.589 0.585 0.103 0.813 表 2 2组老年食管鳞癌患者单因素分析结果
(%) 项目 例数 OS PFS LRFFS 2年 3年 χ2值 P值 2年 3年 χ2值 P值 2年 3年 χ2值 P值 年龄(岁) 4.442 0.035 2.592 0.107 2.402 0.121 ≥76 60 35.3 25.2 33.5 25.8 43.7 33.3 <76 42 58.5 45.6 53.8 38.0 61.2 47.2 性别 0.464 0.496 0.960 0.327 1.294 0.255 男性 68 41.0 31.9 38.6 27.9 48.0 34.6 女性 34 53.8 38.4 48.5 35.3 56.5 48.4 吸烟 0.260 0.610 1.012 0.314 0.382 0.537 有 30 37.4 29.9 32.0 19.2 44.9 26.9 无 72 47.9 35.8 46.2 35.6 53.0 43.8 饮酒 0.566 0.452 1.333 0.248 0.456 0.500 有 23 42.1 31.6 35.4 17.7 51.4 25.7 无 79 45.5 34.3 43.7 34.3 51.1 42.7 原发肿瘤位置 0.784 0.376 1.267 0.260 0.468 0.494 颈、上 33 50.0 45.0 50.8 40.0 55.3 48.4 中、下 69 42.2 26.3 37.3 25.0 48.5 32.5 GTV长度(cm) 3.932 0.047 2.970 0.085 1.673 0.196 ≥6.5 61 36.9 26.8 33.1 23.7 44.4 31.8 <6.5 41 58.5 45.6 56.8 42.3 60.7 50.1 基础疾病 0.902 0.342 0.663 0.415 0.003 0.958 有 39 43.1 30.8 43.0 23.0 58.0 37.3 无 63 46.0 35.8 41.9 33.8 48.5 39.0 PGTV剂量(Gy) 1.048 0.306 2.057 0.151 0.616 0.432 ≥60 85 46.3 36.3 43.8 34.3 53.0 41.4 <60 17 34.9 23.2 33.1 16.5 41.6 31.2 T分期 9.772 0.002 11.276 0.001 9.213 0.002 2~3 55 58.4 41.9 55.4 40.1 62.5 48.8 4 47 29.6 25.4 26.2 18.7 37.2 26.6 N状态 3.943 0.047 6.111 0.013 2.823 0.093 N+ 39 33.5 25.1 28.2 14.1 36.3 24.2 N- 63 51.7 38.8 50.3 39.2 58.8 45.9 临床分期 13.241 < 0.001 14.770 < 0.001 11.366 0.001 Ⅱ~Ⅲ 52 62.0 44.5 58.8 42.5 65.2 50.9 Ⅳa 50 27.9 23.9 24.6 17.6 35.5 25.3 辅助化疗 3.049 0.081 5.097 0.024 8.346 0.004 S-1 42 54.1 42.6 55.4 44.3 68.2 61.4 无 60 38.4 28.5 32.9 21.8 39.2 25.9 表 3 OS独立影响因素的COX多因素回归分析
临床因素 B SE Wald χ2 P值 OR(95% CI) 年龄 0.653 0.298 4.793 0.029 1.922(1.071~3.449) GTV长度 0.154 0.302 0.261 0.610 1.167(0.646~2.108) T分期 -0.677 0.639 1.122 0.289 0.508(0.145~1.778) N状态 0.705 0.285 6.128 0.013 2.024(1.158~3.539) AJCC分期 1.541 0.660 5.441 0.020 4.667(1.279~17.033) 辅助化疗 -0.542 0.287 3.557 0.059 0.582(0.331~1.021) 表 4 PFS独立影响因素的COX多因素回归分析
临床因素 B SE Wald χ2 P值 OR(95% CI) GTV长度 0.179 0.277 0.419 0.518 1.197(0.695~2.060) T分期 -0.557 0.630 0.780 0.377 0.573(0.167~1.971) N状态 0.673 0.265 6.456 0.011 1.960(1.166~3.293) AJCC分期 1.480 0.657 5.072 0.024 4.394(1.212~15.936) 辅助化疗 -0.799 0.272 8.603 0.003 0.450(0.264~0.767) 表 5 LRFFS独立影响因素的COX多因素回归分析
临床因素 B SE Wald χ2 P值 OR(95% CI) T分期 -0.622 0.769 0.655 0.418 0.537(0.119~2.423) N状态 0.570 0.303 3.544 0.060 1.768(0.977~3.201) AJCC分期 1.603 0.792 4.097 0.043 4.968(1.052~23.461) 辅助化疗 -1.094 0.331 10.919 0.001 0.335(0.175~0.641) 表 6 2组老年食管癌患者不良反应发生情况比较
(例) 组别 例数 中性粒细胞减少 血小板减少 贫血 肝功能异常 恶心呕吐 0~1级 ≥2级 0~1级 ≥2级 0~1级 ≥2级 0~1级 ≥2级 0~1级 ≥2级 化疗组 42 38 4 39 3 39 3 40 2 39 3 对照组 60 58 2 58 2 59 1 59 1 60 0 χ2值 0.775 0.169 0.782 0.099 2.268 P值 0.379 0.681 0.377 0.753 0.132 -
[1] BRAY F, FERLAY J, SOERJOMATARAM I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2018, 68(6): 394-424. doi: 10.3322/caac.21492 [2] CHEN W, ZHENG R, BAADE P D, et al. Cancer statistics in China, 2015[J]. CA Cancer J Clin, 2016, 66(2): 115-132. doi: 10.3322/caac.21338 [3] UEDA S, KAWAKAMI H, NISHINA S, et al. Phase Ⅰ trial of 5-FU, docetaxel, and nedaplatin(UDON) combination therapy for recurrent or metastatic esophageal cancer[J]. Cancer Chemoth Pharm, 2015, 76(2): 279-285. doi: 10.1007/s00280-015-2799-3 [4] UEDA H, KAWAKAMI H, NONAGASE Y, et al. Phase Ⅱ trial of 5-Fluorouracil, Docetaxel, and Nedaplatin (UDON) combination therapy for recurrent or metastatic esophageal cancer[J]. Oncologist, 2019, 24(2): 163-176. doi: 10.1634/theoncologist.2018-0653 [5] MBOUMI I W, REDDY S, LIDOR A O. Complications after esophagectomy[J]. Surg Clin North Am, 2019, 99(3): 501-510. doi: 10.1016/j.suc.2019.02.011 [6] GUO J H, CHEN M Q, CHEN C, et al. Efficacy and toxicity of nimotuzumab combined with radiotherapy in elderly patients with esophageal squamous cell carcinoma[J]. Mol Clin Oncol, 2015, 3(5): 1135-1138. doi: 10.3892/mco.2015.606 [7] FAIZ Z, VAN PUTTEN M, VERHOEVEN R H A, et al. Impact of age and comorbidity on choice and outcome of two different treatment options for patients with potentially curable esophageal cancer[J]. Ann Surg Oncol, 2019, 26(4): 986-995. doi: 10.1245/s10434-019-07181-6 [8] HERSKOVIC A, MARTZ K, AL-SARRAF M, et al. Combined chemotherapy and radiotherapy compared with radiotherapy alone in patients with cancer of the esophagus[J]. N Engl J Med, 1992, 326(24): 1593-1598. doi: 10.1056/NEJM199206113262403 [9] ZHANG P, XI M, LI Q Q, et al. Concurrent cisplatin and 5-fluorouracil versus concurrent cisplatin and docetaxel with radiotherapy for esophageal squamous cell carcinoma: a propensity score-matched analysis[J]. Oncotarget, 2016, 7(28): 44686-44694. doi: 10.18632/oncotarget.9301 [10] MIAO Y, ZHAN P, LV T, et al. A meta-analysis of safety and efficacy on first-line S-1 therapy in cancer patients[J]. Transl Lung Cancer Res, 2015, 4(4): 487-497. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4549471/pdf/tlcr-04-04-487.pdf [11] ZHUANG Z X, ZHU H, WANG J, et al. Pharmacokinetic evaluation of novel oral fluorouracil antitumor drug S-1 in Chinese cancer patients[J]. Acta Pharmacol Sin, 2013, 34(4): 570-580. doi: 10.1038/aps.2012.169 [12] KASAI T, MORI K, KISHI K, et al. A phase Ⅰ and extension study of S-1 and carboplatin for previously untreated patients aged 75 years or more with advanced non-small cell lung cancer -TCOG 1101-[J]. Int J Clin Oncol, 2020, 25(24): 867-875. [13] CHEN J, WANG J. Efficacy and safety assessment of S-1-based regimens comparing to intravenous fluorouracil-based ones in Asian patients with metastatic colorectal carcinoma[J]. Medicine, 2019, 98(23): e15999. doi: 10.1097/MD.0000000000015999 [14] LI W, ZHAO X, WANG H, et al. Maintenance treatment of Uracil and Tegafur(UFT) in responders following first-line fluorouracil-based chemotherapy in metastatic gastric cancer: a randomized phase Ⅱ study[J]. Oncotarget, 2017, 8(23): 37826-37834. doi: 10.18632/oncotarget.13922 [15] AKIYAMA N, KARAYAMA M, INUI N, et al. Switch maintenance therapy with S-1 after induction therapy with carboplatin and nanoparticle albumin-bound paclitaxel in advanced lung squamous cell carcinoma[J]. Invest New Drugs, 2019, 37(3): 531-537. doi: 10.1007/s10637-019-00747-x [16] SONG T, LV S, FANG M, et al. Long-term results of definitive concurrent chemoradiotherapy using S-1 in the treatment of geriatric patients with esophageal cancer[J]. Onco Targets Ther, 2016, 9: 5389-5397. doi: 10.2147/OTT.S107668 [17] SHAPIRO J, VAN LANSCHOT J, HULSHOF M, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer(CROSS): long-term results of a randomised controlled trial[J]. Lancet Oncol, 2015, 16(9): 1090-1098. doi: 10.1016/S1470-2045(15)00040-6 [18] VONCKEN F, VAN DER KAAIJ R T, SIKORSKA K, et al. Advanced age is not a contraindication for treatment with curative intent in esophageal cancer[J]. Am J Clin Oncol, 2018, 41(9): 919-926. doi: 10.1097/COC.0000000000000390 -





 下载:
下载: