PM-induced IL-17A expression enhances airway inflammation in cigarette-exposed mice
-
摘要:
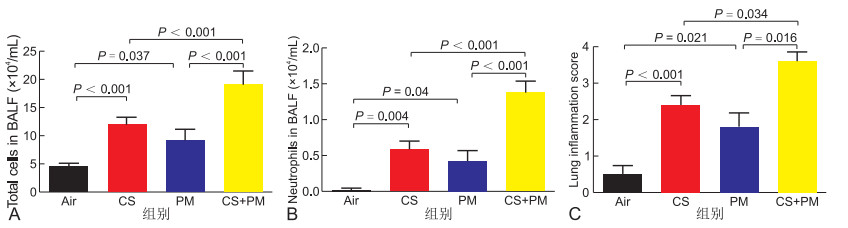
目的 探讨大气细颗粒物(PM)增强香烟暴露小鼠炎症反应的机制。 方法 用野生型(WT)及IL-17A基因敲除(IL-17A-/-)小鼠,按随机数字表法随机分为对照组、熏烟组、PM组、熏烟+PM组,每组6~8只。采用香烟烟雾暴露装置烟熏,气道滴注方法吸入PM,观察气道炎症反应,连续干预3个月后取检。用HE检测肺组织炎症浸润、用ELISA及RT-PCR检测肺组织炎症因子表达、免疫组化检测平滑肌表达、Masson染色观察胶原沉积及细胞流式检测分泌IL-17A的淋巴细胞类型。体外实验,用不同浓度的香烟提取物(CSE)和/或大气细微颗粒(PM)刺激人气道上皮(HBE)细胞,用IL-17A siRNA转染在HBE细胞中敲除IL-17A基因,用RT-PCR检测炎症因子表达。 结果 与对照组、熏烟组及PM组比较,熏烟联合PM组小鼠肺组织炎症因子(CXCL1、TFG-β1、IL-6及IL-17A)、胶原沉积及平滑肌表达明显增高。相反,IL-17A-/-小鼠能缓解上述指标。流式细胞检测发现PM主要通过调控CD4+细胞促进IL-17A表达,增强熏烟诱导的炎症反应。在体外,CSE、PM分别干预HBE细胞均能诱导IL-6、IL-8表达,而CSE联合PM干预HBE细胞能进一步增加IL-6、IL-8表达。敲除HBE细胞IL-17A基因后,能缓解IL-6、IL-8表达。 结论 PM能诱导IL-17A表达,加剧熏烟小鼠肺组织的炎症反应、胶原沉积及平滑肌增生,提示针对IL-17A信号通路靶向治疗可能对缓解PM导致的慢性阻塞性肺疾病急性加重有效。 Abstract:Objective This study aimed to investigate the mechanisms of atmospheric fine particulate matter (PM), which enhanced the inflammatory response in cigarette-exposed mice. Methods Wild-type and IL-17A knockout (IL-17A-/-) mice were randomly divided into the control group, smoked group, PM group and smoked+PM group according to the random number table method (6-8 mice/group). Mice were exposed to smoke using cigarette smoke exposure device. PM was inhaled by airway instillation, and airway inflammation was observed. After continuous intervention for 3 months, mice were sacrificed to measure airway inflammation. HE was used to detect inflammatory infiltration in lung tissue, and ELISA and RT-PCR were used to detect inflammatory cytokines in lung tissue. Smooth muscle expression was detected by immunohistochemistry, and collagen deposition was observed by Masson staining. In addition, IL-17A-secreting lymphocytes were detected by cell flow cytometry. In in vitro experiments, different concentrations of cigarette smoke extract (CSE) and/or PM were used to stimulate human airway epithelial (HBE) cells, and IL-17A siRNA was used to knock down IL-17A gene in HBE cells. RT-PCR detects the expression of inflammatory factors. Results Compared with the control, smoked and PM groups, lung tissue inflammatory factors (CXCL1, TFG-β1, IL-6 and IL-17A), collagen deposition and smooth muscle expression were significantly increased in the smoked+PM group. By contrast, IL-17A-/- mice can alleviate the above-mentioned indicators. Flow cytometry found that PM promoted IL-17A expression by regulating CD4 cells and worsened the inflammatory response induced by smoking. In vitro, CSE or PM intervention in HBE cells can induce the secretion of IL-6 and IL-8, whereas CSE combined with PM can further increase the expression of IL-6 and IL-8. Knockout of IL-17A gene in HBE cells can alleviate the expression of IL-6 and IL-8. Conclusion PM can induce the expression of IL-17A and exacerbate the inflammation, collagen deposition and smooth muscle hyperplasia in the lung tissue of cigarette-exposed mice, suggesting that targeted therapy for the IL-17A signalling pathway may be effective in relieving acute exacerbation of COPD caused by PM. -
Key words:
- Particulate matter /
- Airway inflammation /
- IL-17A
-
表 1 引物序列
项目 类别 IL-1β(鼠) Forward Primer GAAATGCCACCTTTTGACAGTG Reverse Primer TGGATGCTCTCATCAGGACAG IL-6(鼠) Forward Primer CTGCAAGAGACTTCCATCCAG Reverse Primer AGTGGTATAGACAGGTCTGTTGG IL-17A(鼠) Forward Primer TCAGCGTGTCCAAACACTGA Reverse Primer CGCCAAGGGAGTTAAAGACTT GAPDH(鼠) Forward Primer GGGTTCCTATAAATACGGACTGC Reverse Primer CCATTTTGTCTACGGGACGA IL-6(人) Forward Primer TGATGGATGCTACCAAACTGG Reverse Primer TTCATGTACTCCAGGTAGCTATGG IL-8(人) Forward Primer ACTGAGAGTGATTGAGAGTGGAC Reverse Primer AACCCTCTGCACCCAGTTTTC GAPDH(人) Forward Primer TGTTGCCATCAATGACCCCTT Reverse Primer CTCCACGACGTACTCAGCG -
[1] WANG C, XU J, YANG L, et al. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China Pulmonary Health[CPH] study): a national cross-sectional study[J]. Lancet, 2018, 391(10131): 1706-1717. doi: 10.1016/S0140-6736(18)30841-9 [2] WANG M, AARON C P, MADRIGANO J, et al. Association between long-term exposure to ambient air pollution and change in quantitatively assessed emphysema and lung function[J]. JAMA, 2019, 322(6): 546-556. doi: 10.1001/jama.2019.10255 [3] HUANG S, GARSHICK E, VIEIRA C L Z, et al. Short-term exposures to particulate matter gamma radiation activities and biomarkers of systemic inflammation and endothelial activation in COPD patients[J]. Environ Res, 2020, 180: 108841. doi: 10.1016/j.envres.2019.108841 [4] WU Y F, LI Z Y, DONG L L, et al. Inactivation of MTOR promotes autophagy-mediated epithelial injury in particulate matter-induced airway inflammation[J]. Autophagy, 2020, 16(3): 435-450. doi: 10.1080/15548627.2019.1628536 [5] PONCE-GALLEGOS M A, PÉREZ-RUBIO G, AMBROCIO-ORTIZ E, et al. Genetic variants in IL17A and serum levels of IL-17A are associated with COPD related to tobacco smoking and biomass burning[J]. Sci Rep, 2020, 10(1): 784. doi: 10.1038/s41598-020-57606-6 [6] ZHOU J S, LI Z Y, XU X C, et al. Cigarette smoke-initiated autoimmunity facilitates sensitisation to elastin-induced COPD-like pathologies in mice[J]. Eur Respir J, 2020, 56(3): 2000404. doi: 10.1183/13993003.00404-2020 [7] LI H, YAN X, FENG S, et al. Antagonism of interleukin 17 protects chronic obstructive pulmonary disease rat lungs from adverse effects of environmental PM (2.5)[J]. Am J Transl Res, 2020, 12(9): 5808-5817. [8] CHRISTENSON S A, VAN DEN BERGE M, FAIZ A, et al. An airway epithelial IL-17A response signature identifies a steroid-unresponsive COPD patient subgroup[J]. J Clin Invest, 2019, 129(1): 169-181. http://www.onacademic.com/detail/journal_1000041647909299_8ab8.html [9] YU Y, ZHAO L, XIE Y, et al. Th1/Th17 Cytokine Profiles are Associated with Disease Severity and Exacerbation Frequency in COPD Patients[J]. Int J Chron Obstruct Pulmon Dis, 2020, 15: 1287-1299. doi: 10.2147/COPD.S252097 [10] OUYANG S, LIU C, XIAO J, et al. Targeting IL-17A/glucocorticoid synergy to CSF3 expression in neutrophilic airway diseases[J]. JCI Insight, 2020, 5(3): e132836. doi: 10.1172/jci.insight.132836 [11] LAI T, TIAN B, CAO C, et al. HDAC2 Suppresses IL17A-mediated airway remodeling in human and experimental modeling of COPD[J]. Chest, 2018, 153(4): 863-875. doi: 10.1016/j.chest.2017.10.031 [12] LAI T, WU M, ZHANG C, et al. HDAC2 attenuates airway inflammation by suppressing IL-17A production in HDM-challenged mice[J]. Am J Physiol Lung Cell Mol Physiol, 2019, 316(1): L269-L279. doi: 10.1152/ajplung.00143.2018 [13] HUANG S, GARSHICK E, VIEIRA C L Z, et al. Short-term exposures to particulate matter gamma radiation activities and biomarkers of systemic inflammation and endothelial activation in COPD patients[J]. Environ Res, 2020, 180: 108841. doi: 10.1016/j.envres.2019.108841 [14] LIANG L, CAI Y, BARRATT B, et al. Associations between daily air quality and hospitalisations for acute exacerbation of chronic obstructive pulmonary disease in Beijing, 2013-17: an ecological analysis[J]. Lancet Planet Health, 2019, 3(6): e270-e279. doi: 10.1016/S2542-5196(19)30085-3 [15] MORANTES-CABALLERO J A, FAJARDO RODRIGUEZ H A. Effects of air pollution on acute exacerbation of chronic obstructive pulmonary disease: a descriptive retrospective study (pol-AECOPD)[J]. Int J Chron Obstruct Pulmon Dis, 2019, 14: 1549-1557. doi: 10.2147/COPD.S192047 -





 下载:
下载: