Study on the inhibitory effects of miR-140-5p targeting YES1 proto-oncogene
-
摘要:
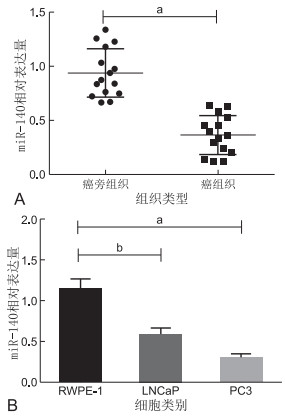
目的 研究miR-140-5p与YES1的靶向调控关系,阐明miR-140-5p通过YES1调控前列腺癌发生发展的作用,并研究miR-140-5p在前列腺癌细胞中的潜在作用机制。 方法 采用实时定量PCR检测前列腺癌组织和细胞系中miR-140-5p的表达,采用CCK-8、细胞克隆实验、迁移和划痕实验测定miR-140-5p上调对前列腺癌细胞增殖和迁移的影响,用荧光素酶报告实验和蛋白印迹实验鉴定miR-140-5p的靶基因。 结果 癌组织中的miR-140-5p表达量相对癌旁组织明显下调,差异有统计学意义(P<0.05)。与RWPE-1细胞相比,前列腺癌细胞系中miR-140-5p的表达水平较低,差异具有统计学意义(P<0.05)。CCK-8实验转染后miR-140-5p的细胞增殖率显著下降,细胞克隆实验实验组相比对照组细胞克隆数目偏少,差异均有统计学意义(P<0.05)。划痕实验转染miR-140-5p细胞系的愈合能力明显减弱(P < 0.05);Transwell小室实验转染miR-140-5p的细胞系的迁移能力下降(P < 0.05);说明过表达miR-140-5p可抑制细胞的增殖和迁移。生物信息学分析和荧光素酶报告分析确定YES1为miR-140-5p的潜在靶基因。YES1在前列腺癌细胞中过表达,与miR-140-5p表达呈负相关,差异具有统计学意义(P<0.05)。 结论 以上实验表明miR-140-5p通过直接靶向YES1在前列腺癌发生发展中起抑制作用,提示miR-140-5p可能是一个新的靶点,用于前列腺癌的诊断和治疗。 -
关键词:
- miR-140-5p /
- 前列腺癌 /
- 迁移 /
- 增殖
Abstract:Objective To study the targeted regulatory relationship between miR-140-5p and YES1 and elucidate the role of miR-140-5p in regulating the occurrence and development of prostate cancer through YES1. The potential mechanism of action of miR-140-5p in prostate cancer cells was also determined. Methods The expression of miR-140-5p in prostate cancer tissues and cell lines was detected via real-time quantitative PCR. The effects of miR-140-5p up-regulation on the proliferation and migration of prostate cancer cells were investigated via cell Counting Kit-8 (CCK-8) assay, cell cloning experiment, and migration and scratch assay. The target gene of miR-140-5p was identified via luciferase reporter assays and Western blotting. Results The expression level of miR-140-5p in cancer tissues was significantly down-regulated compared with that in adjacent tissues (P < 0.05). The expression level of miR-140-5p was significantly lower in prostate cancer cell lines than in RWPE-1 cells (P < 0.05). After transfection via CCK-8 assay, the proliferation rate of cells transfected with miR-140-5p significantly decreased, and the number of clones in the hyroid cell clone experiment group was significantly lower than that in the control group (P < 0.05). The healing ability of cell lines transfected with miR-140-5p in the scratch experiments significantly decreased (P < 0.05). Transwell assay revealed that the migration ability of cell lines transfected with miR-140-5p (P < 0.05) decreased, suggesting that overexpression of miR-140-5p can inhibit cell proliferation and migration. Bioinformatics analysis and luciferase report analysis identified YES1 as the potential target gene of miR-140-5p. YES1 overexpression in prostate cancer cells was negatively correlated with miR-140-5p expression, and the difference was statistically significant (P < 0.05). Conclusion These experiments demonstrated that miR-140-5p can inhibit the development of prostate cancer by directly targeting YES1, suggesting that miR-140-5p may be a new target for the diagnosis and treatment of prostate cancer. -
Key words:
- MiR-140-5p /
- Prostate cancer /
- Migration /
- Proliferation
-
表 1 15例前列腺癌患者miR-140-5p对比(x ±s)
项目 类别 例数 miR-140-5p 统计量 P值 年龄(岁) 50~60 2 0.49±0.07 0.313a 0.757 61~70 5 0.46±0.08 71~79 8 0.48±0.08 Gleason分级 ≤6 3 0.52±0.07 5.274a 0.037 7 6 0.46±0.09 ≥8 6 0.43±0.08 肿瘤直径(cm) <3 6 0.48±0.08 1.427b 0.159 ≥3 9 0.46±0.08 组织分化程度 高分化 5 0.49±0.09 7.643a 0.014 中分化 7 0.44±0.07 低分化 3 0.42±0.07 注:a为F值,b为t值。 -
[1] GUPTA S, PUNGSRINONT T, ZENATA O, et al. Interleukin-23 represses the level of cell senescence induced by the androgen receptor antagonists enzalutamide and darolutamide in castration-resistant prostate cancer cells[J]. Horm Cancer, 2020, 11(3-4): 182-190. doi: 10.1007/s12672-020-00391-5 [2] MORRISON B F, AIKEN W D, MAYHEW R. Current state of prostate cancer treatment in Jamaica[J]. Ecancermedicalscience, 2014, 8: 456. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4154943/pdf/can-8-456.pdf [3] KIENER M, CHEN L P, KREBS M, et al. miR-221-5p regulates proliferation and migration in human prostate cancer cells and reduces tumor growth in vivo[J]. BMC Cancer, 2019, 19(1): 627. doi: 10.1186/s12885-019-5819-6 [4] VILA-NAVARRO E, FERNANDEZ-CASTANER E, ROVIRA-RIGAU M, et al. MiR-93 is related to poor prognosis in pancreatic cancer and promotes tumor progression by targeting microtubule dynamics[J]. Oncogenesis, 2020, 9(5): 281-297. http://www.nature.com/articles/s41389-020-0227-y [5] SUN X L, LI Y K, YU J, et al. miR-128 modulates chemosensitivity and invasion of prostate cancer cells through targeting ZEB1[J]. Jpn J Clin Oncol, 2015, 45(5): 474-482. doi: 10.1093/jjco/hyv027 [6] BASKERVILLE S, BARTEL D P. Microarray profiling of microRNAs reveals frequent coexpression with neighboring miRNAs and host genes[J]. RNA, 2005, 11(3): 241-247. doi: 10.1261/rna.7240905 [7] URBANEK-TRZECIAK M O, JAWORSKA E, KRZYZOSIAK W J. miRNAmotif-a tool for the prediction of pre-miRNA-protein interactions[J]. Int J Mol Sci, 2018, 19(12): 4075. doi: 10.3390/ijms19124075 [8] WEI L Z, WANG Y Q, CHANG Y L, et al. Imbalance of a KLF4-miR-7 auto-regulatory feedback loop promotes prostate cancer cell growth by impairing microRNA processing[J]. Am J Cancer Res, 2018, 8(2): 226-244. http://ajcr.us/files/ajcr0068245.pdf [9] WEGERT J, ISHAQUE N, VARDAPOUR R, et al. Mutations in the SIX1/2 pathway and the DROSHA/DGCR8 miRNA microprocessor complex underlie high-risk blastemal type Wilms tumors[J]. Cancer Cell, 2015, 27(2): 298-311. doi: 10.1016/j.ccell.2015.01.002 [10] BARTEL D P. MicroRNAs: target recognition and regulatory functions[J]. Cell, 2009, 136(2): 215-233. doi: 10.1016/j.cell.2009.01.002 [11] YU A, ZHANG J, MEI Y, et al. Correlation between single nucleotide polymorphisms of an miRNA binding site in the 3'UTR of PTEN and risk of cervical cancer among the Han Chinese[J]. Genet Test Mol Biomarkers, 2020, 24(7): 381-389. doi: 10.1089/gtmb.2019.0269 [12] YANG B, MCJUNKIN K. The mir-35-42 binding site in the nhl-2 3'UTR is dispensable for development and fecundity[J]. MicroPubl Biol, 2020. DOI: 10.17912/micropub.biology.000241. [13] JONES D Z, SCHMIDT M L, SUMAN S, et al. Micro-RNA-186-5p inhibition attenuates proliferation, anchorage independent growth and invasion in metastatic prostate cancer cells[J]. BMC Cancer, 2018, 18(1): 421. doi: 10.1186/s12885-018-4258-0 [14] MORIDIKIA A, MIRZAEI H, SAHEBKAR A, et al. MicroRNAs: Potential candidates for diagnosis and treatment of colorectal cancer[J]. J Cell Physiol, 2018, 233(2): 901-913. doi: 10.1002/jcp.25801 [15] FAN H, ZHANG Y S. miR-490-3p modulates the progression of prostate cancer through regulating histone deacetylase 2[J]. Eur Rev Med Pharmacol Sci, 2019, 23(2): 539-546. http://www.researchgate.net/publication/331147580_MiR-490-3p_modulates_the_progression_of_prostate_cancer_through_regulating_histone_deacetylase_2 [16] BAI M H, LEI Y, WANG M C, et al. Long non-coding RNA SNHG17 promotes cell proliferation and invasion in castration-resistant prostate cancer by targeting the miR-144/CD51 axis[J]. Front Genet, 2020, 11: 274. doi: 10.3389/fgene.2020.00274 [17] GUPTA S, SILVEIRA D A, MOMBACH J C M. Modeling the role of microRNA-449a in the regulation of the G2/M cell cycle checkpoint in prostate LNCaP cells under ionizing radiation[J]. PLoS One, 2018, 13(7): e0200768. doi: 10.1371/journal.pone.0200768 [18] HAO S D, MA J X, LIU Y, et al. Long non-coding TUG1 accelerates prostate cancer progression through regulating miR-128-3p/YES1 axis[J]. Eur Rev Med Pharmacol Sci, 2020, 24(2): 619-632. http://www.researchgate.net/publication/339067247_Long_non-coding_TUG1_accelerates_prostate_cancer_progression_through_regulating_miR-128-3pYES1_axis [19] ELISI G M, SANTICCI M, DARCA D, et al. Repurposing of drugs targeting YAP-TEAD functions[J]. Cancers(Basel), 2018, 10(9): 329. http://www.mdpi.com/2072-6694/10/9/329/pdf [20] HAMANAKA N, NAKANISHI Y, MIZUNO T, et al. YES1 is a targetable oncogene in cancers harboring YES1 gene amplification[J]. Cancer Res, 2019, 79(22): 5734-5745. doi: 10.1158/0008-5472.CAN-18-3376 [21] ROSENBLUH J, NIJHAWAN D, COX A G, et al. β-Catenin-driven cancers require a YAP1 transcriptional complex for survival and tumorigenesis[J]. Cell, 2012, 151(7): 1457-1473. doi: 10.1016/j.cell.2012.11.026 [22] TAN W, LIM S G, TAN T M. Up-regulation of microRNA-210 inhibits proliferation of hepatocellular carcinoma cells by targeting YES1[J]. World J Gastroenterol, 2015, 21(46): 13030-13041. doi: 10.3748/wjg.v21.i46.13030 [23] ZHANG G, WANG J, ZHENG R, et al. MiR-133 targets YES1 and inhibits the growth of triple-negative breast cancer cells[J]. Technol Cancer Res Treat, 2020. DOI: 10.1177/1533033820927011 [24] YE P, LV X, AIZEMAITI R, et al. H3K27ac-activated LINC00519 promotes lung squamous cell carcinoma progression by targeting miR-450b-5p/miR-515-5p/YAP1 axis[J]. Cell Prolif, 2020, 53(5): e12797. doi: 10.1111/cpr.12797 -





 下载:
下载: