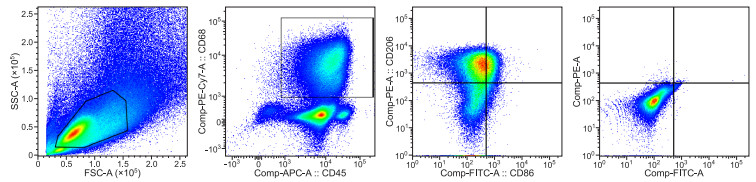
Alveolar macrophage phenotypes in bronchoalveolar lavage fluid from sepsis
-
摘要:
目的 脓毒症是一种死亡率较高的感染性疾病,以免疫紊乱为特征。巨噬细胞作为一种重要的抗原提呈细胞,在先天免疫和获得性免疫中发挥不可或缺的作用, 其接受不同刺激时可分化为不同的表型——M1和M2,分别具有促进炎症、组织修复重塑的功能。本研究拟观察脓毒症患者支气管肺泡灌洗液(BALF)中肺泡巨噬细胞(alveolar macrophage,AM)表型变化,并分析其不同表型与脓毒症患者预后的关系。 方法 选取2020年7—10月重症医学科(ICU)和呼吸与危重症医学科(PCCM)病房中29例脓毒症患者,分别于第1天和第7天行支气管肺泡灌洗(bronchoalveolar lavage, BAL)。采用CD68、CD86和CD206三种流式抗体检测AM在BALF中的比例。以CD68抗体作为AM的标记,CD86和CD206抗体分别作为M1和M2标记。同时收集基础临床资料,根据28 d病死率将受试者分为存活组(20例)和死亡组(9例)。 结果 流式细胞术显示M1和M2型AM的形态结构不同,M1的细胞大小和细胞颗粒复杂度均大于M2。存活组第1天BALF中M1和M2分别为(21.39±9.91)%、(4.66±2.53)%,第7天分别为(3.62±3.31)%、(44.41±7.47)%,M1比例较第1天明显下降且M2比例明显上升,差异有统计学意义(均P < 0.05);死亡组第1天M1和M2分别为(18.37±2.75)%、(20.40±2.27)%,第7天分别为(7.00±1.98)%、(19.69±4.24)%,M1比例较第1天下降(P < 0.05)且M2比例持续高于M1,M2比例第7天与第1天比较差异无统计学意义(P>0.05)。 结论 早期动态监测肺泡巨噬细胞表型变化,可能有助于预测脓毒症患者28 d内预后情况。 Abstract:Objective Sepsis is an immune disorder and an infectious disease with high mortality. As important antigen-presenting cells, macrophages play an indispensable role in innate immunity and adaptive immunity. Macrophages may polarise into two distinct phenotypes presenting as M1 and M2 when cells accept different stimuli. M1 macrophages and M2 macrophages enhance inflammation and tissue repair and remodelling, respectively. This study aimed to observe changes in alveolar macrophage (AMs) phenotypes in the bronchoalveolar lavage fluid (BALF) of patients with sepsis and analyse the correlation between the two distinct AM phenotypes and prognosis of patients with sepsis. Methods Twenty-nine patients who experienced consecutive sepsis and admitted to the intensive care unit of the Department of Pulmonary and Critical Care Medicine from July 2020 to December 2020 were included in this study. These patients underwent bronchoalveolar lavage on the first and the seventh days after the admission. The proportion of AMs in BALF was assessed via flow cytometry by using three colour fluorescent stains. CD68 was used as the marker of, total AM whereas CD86 and CD206 were used as M1 and M2 AM markers, respectively. The basic clinical data of the patients were collected and analysed. They were divided into a survival group and a non-survivor group according to mortality data within 28 days. Results The morphological structures of the M1 and M2 AMs were different. Flow cytometry revealed that the M1 AMs have a larger cell size and granularity than the M1 AMs. In the survivor group, the proportion of M1 and M2 in BALF were (21.39±9.91)% and (4.66±2.53)%, respectively, on the first day. On the seventh day, the proportion of M1 and M2 in BALF were (3.62±3.31)% and (44.41±7.47)%, respectively. The percentage of M2 and M1 AMs from BALF increased and decreased, respectively, on the seventh day compared with that on the first day (all P < 0.05). In the nonsurvivor group, the proportion of M1 and M2 in BALF were (18.37±2.75)% and (20.40±2.27)%, respectively, on the first day and (7.00±1.98)% and (19.69±4.24)%, respectively, on the seventh day., the percentage of M1 decreased, whereas that of M2 continued to increase compared with that of M1 (all P < 0.05). No statistically significant differences were observed between the first and the seventh days (P>0.05). Conclusion Early dynamic monitoring of changes in AM phenotypes can help predict the prognosis of patients with sepsis within 28 days. -
Key words:
- Sepsis /
- Alveolar macrophages /
- Antigen presenting cell /
- Bronchoalveolar lavage fluid /
- Flow cytometry
-
表 1 存活组与死亡组脓毒症患者M1和M2型AM比例比较(x±s, %)
组别 例数 M1 M2 第1天 第7天 第1天 第7天 存活组 20 21.39±9.91 3.62±3.31a 4.66±2.53 44.41±7.47a 死亡组 9 18.37±2.75 7.00±1.98a 20.40±2.27 19.69±4.24 t值 1.261 -2.825 -15.966 9.222 P值 0.219 0.009 < 0.001 < 0.001 注:与组内第1天比较,aP < 0.05。 -
[1] 钱幸尔, 郑旻, 戴晓薇, 等. 脓毒症患者并发医院感染和死亡的危险因素分析[J]. 中华全科医学, 2018, 16(2): 232-235. https://www.cnki.com.cn/Article/CJFDTOTAL-SYQY201802020.htm [2] REINHART K, DANIELS R, KISSOON N, et al. Recognizing sepsis as a global health priority-A WHO Resolution[J]. N Engl J Med, 2017, 377(5): 414-417. doi: 10.1056/NEJMp1707170 [3] RUDD K E, JOHNSON S C, AGESA K M, et al. Global, regional, and national sepsis incidence and mortality, 1990-2017: analysis for the global burden of disease study[J]. Lancet, 2020, 395(10219): 200-211. doi: 10.1016/S0140-6736(19)32989-7 [4] SINGER M, DEUTSCHMAN C S, SEYMOUR C W, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3)[J]. JAMA, 2016, 315(8): 801-810. doi: 10.1001/jama.2016.0287 [5] ZHAO J, YU H, LIU Y, et al. Protective effect of suppressing STAT3 activity in LPS-induced acute lung injury[J]. Am J Physiol Lung Cell Mol Physiol, 2016, 311(5): 868-880. doi: 10.1152/ajplung.00281.2016 [6] SAFAVIAN D, LEUNG C H, KAPUS A, et al. Hemorrhagic shock/resuscitation reduces the M2 phenotype of alveolar macrophages: a potential mechanism contributing to increased LPS-induced lung injury[J]. Shock, 2019, 51(2): 213-220. doi: 10.1097/SHK.0000000000001135 [7] ALLARD B, PANARITI A, MARTIN J G. Alveolar macrophages in the resolution of inflammation, tissue repair, and tolerance to infection[J]. Front Immunol, 2018, 9: 1777. doi: 10.3389/fimmu.2018.01777 [8] MUXEL S M, AOKI J I, FERNANDES J C R, et al. Arginine and Polyamines Fate in Leishmania Infection[J]. Fron Microbiol, 2017, 8: 2628. doi: 10.3389/fmicb.2017.02682/pdf [9] 中华医学会呼吸病学分会介入呼吸病学学组. 成人诊断性可弯曲支气管镜检查术应用指南(2019年版)[J]. 中华结核和呼吸杂志, 2019, 42(8): 573-590. https://www.cnki.com.cn/Article/CJFDTOTAL-HBYX202011001.htm [10] SANJURJO L, ARAN G, T LLEZ É, et al. CD5L promotes M2 macrophage polarization through autophagy-mediated upregulation of ID3[J]. Front Immunol, 2018, 9: 480. doi: 10.3389/fimmu.2018.00480 [11] BINNIE A, TSANG J L Y, HU P, et al. Epigenetics of sepsis[J]. Crit Care Med, 2020, 48(5): 745-756. doi: 10.1097/CCM.0000000000004247 [12] DELANO M J, WARD P A. The immune system's role in sepsis progression, resolution, and long-term outcome[J]. Immunol Rev, 2016, 274(1): 330-353. doi: 10.1111/imr.12499 [13] ATRI C, GUERFALI F Z, LAOUINI D. Role of human macrophage polarization in inflammation during infectious diseases[J]. Int J Mol Sci, 2018, 19(6): 1801. doi: 10.3390/ijms19061801 [14] ROQUILLY A, MCWILLIAM H E G, JACQUELINE C, et al. Local modulation of antigen-presenting cell development after resolution of pneumonia induces long-term susceptibility to secondary infections[J]. Immunity, 2017, 47(1): 135-147. doi: 10.1016/j.immuni.2017.06.021 [15] GONG W, ZHU H, LU L, et al. A benzenediamine analog Fc-99 drives M2 macrophage polarization and alleviates lipopolysaccharide-(LPS-) induced liver injury[J]. Mediators Inflamm, 2019, 2019: 7823069. http://www.researchgate.net/publication/334815042_A_Benzenediamine_Analog_FC-99_Drives_M2_Macrophage_Polarization_and_Alleviates_Lipopolysaccharide-_LPS-_Induced_Liver_Injury [16] 陈祝桂, 彭志勇, 张智豪, 等. 不同浓度脂多糖对脓毒症急性肺损伤肺上皮细胞坏死性凋亡和线粒体自噬的影响[J]. 中华实用诊断与治疗杂志, 2020, 34(4): 330-333. https://www.cnki.com.cn/Article/CJFDTOTAL-HNZD202004002.htm [17] LIU M, CHEN Y, WANG S, et al. α-ketoglutarate modulates macrophage polarization through regulation of PPARγ transcription and MTORC1/P70S6K pathway to ameliorate ALI/ARDS[J]. Shock, 2020, 53(1): 103-113. doi: 10.1097/SHK.0000000000001333 [18] GARNIER M, GIBELIN A, MAILLEUX A A, et al. Macrophage polarization favors epithelial repair during acute respiratory distress syndrome[J]. Crit Care Med, 2018, 46(7): 692-701. doi: 10.1097/CCM.0000000000003150 [19] HU L, CHEN Z, LI L, et al. Resveratrol decreases CD45(+) CD206(-) subtype macrophages in LPS-induced murine acute lung injury by SOCS3 signalling pathway[J]. J Cell Mol Med, 2019, 23(12): 8101-8113. doi: 10.1111/jcmm.14680 [20] SHEN Y, SONG J, WANG Y, et al. M2 macrophages promote pulmonary endothelial cells regeneration in sepsis-induced acute lung injury[J]. Ann Transl Med, 2019, 7(7): 142. doi: 10.21037/atm.2019.02.47 [21] JOSHI N, WALTER J M, MISHARIN A V. Alveolar macrophages[J]. Cell Immunol, 2018, 330: 86-90. doi: 10.1016/j.cellimm.2018.01.005 [22] DELANO M J, WARD P A. Sepsis-induced immune dysfunction: can immune therapies reduce mortality?[J]. J Clin Invest, 2016, 126(1): 23-31. doi: 10.1172/JCI82224 [23] SONG C, LI H, LI Y, et al. NETs promote ALI/ARDS inflammation by regulating alveolar macrophage polarization[J]. Exp Cell Res, 2019, 382(2): 111486. doi: 10.1016/j.yexcr.2019.06.031 [24] HOTCHKISS R S, MOLDAWER L L, OPAL S M, et al. Sepsis and septic shock[J]. Nat Rev Dis Primers, 2016, 2: 16045. doi: 10.1038/nrdp.2016.45 [25] WATANABE N, SUZUKI Y, INOKUCHI S, et al. Sepsis induces incomplete M2 phenotype polarization in peritoneal exudate cells in mice[J]. J Intensive Care, 2016, 4(1): 6. doi: 10.1186/s40560-015-0124-1 [26] TARATUMMARAT S, SANGPHECH N, VU C T B, et al. Gold nanoparticles attenuates bacterial sepsis in cecal ligation and puncture mouse model through the induction of M2 macrophage polarization[J]. BMC Microbiol, 2018, 18(1): 85. doi: 10.1186/s12866-018-1227-3 -





 下载:
下载: