Effect of intermittent θ pulse stimulation on frequency spectrum and frequency ratio of EEG in patients with type 2 diabetes mellitus and mild cognitive impairment
-
摘要:
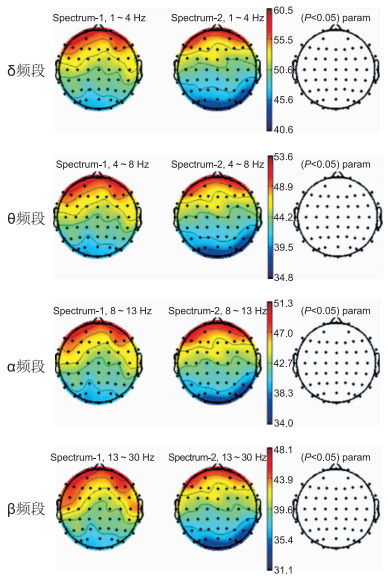
目的 探讨单次间歇性θ脉冲刺激对2型糖尿病合并轻度认知功能障碍患者静息脑电图频率谱与频率比的影响。 方法 2020年7—11月在深圳宝安医院下属社区卫生服务中心招募40例2型糖尿病合并轻度认知功能障碍患者,按随机数字表法分为真刺激组(20例)和假刺激组(20例)。每位患者首先接受5 min静息脑电图采集,再用单脉冲测量静息运动阈值,以80%强度进行一次间歇性θ脉冲刺激,刺激后立即再次进行5 min静息脑电图采集。 结果 间歇性θ脉冲刺激干预后,假刺激组干预前后各电极通道各频段的绝对功率差异无统计学意义。假刺激组和真刺激组刺激后各电极通道各频段的绝对功率差异无统计学意义。但真刺激组干预后与刺激前相比,T7和CP6通道的β频段绝对功率显著增高,分别为(45.83±8.28)μV2、(39.84±11.10)μV2,差异有统计学意义(P=0.042、P=0.040)。与刺激前相比,真刺激组刺激后T7通道的(δ+θ)/(α+β)频率比显著降低,差异有统计学意义(P=0.041)。且经多因素回归分析发现,受试者的性别、年龄、体重指数和病程与绝对功率的变化差异无统计学意义(均P > 0.05)。 结论 单次间歇性θ脉冲刺激干预后,可提高2型糖尿病合并轻度认知功能障碍患者颞区T7和CP6通道β频段的绝对功率,并降低T7通道的频率比。iTBS通过影响2型糖尿病合并轻度认知功能障碍患者的认知加工过程,从而改善认知功能。 Abstract:Objective To investigate the effect of single intermittent θ pulse stimulation on the frequency spectrum and frequency ratio of resting EEG in patients with type 2 diabetes mellitus and mild cognitive impairment. Methods From July to November 2020, 40 patients with type 2 diabetes mellitus complicated with mild cognitive impairment were recruited in the community health service center affiliated to Shenzhen Bao 'an Hospital. They were randomly divided into true stimulation group (n=20) and sham stimulation group (n=20). Each patient received a 5 min resting EEG collection first, with a single pulse measurement resting movement threshold, an intermittent θ pulse was stimulated at 80% intensity, and EEG was collected at rest for 5min immediately after stimulation. Results After the intervention of intermittent θ pulse stimulation, there was no significant difference in the absolute power of each electrode channel and each frequency band before and after the intervention of sham stimulation group. There was no significant difference in the absolute power of each frequency band of the electrode channel between the sham stimulus group and the true stimulus group. However, in the true stimulation group, the absolute β-band power of T7 and CP6 channels increased significantly after intervention compared with before stimulation, respectively (45.83±8.28) μV2, (39.84±11.10) μV2, the difference was statistically significant (P=0.042, P=0.040). Compared with before stimulation, the (δ+θ)/(α+β) frequency ratio of T7 channel in the true stimulation group decreased significantly after stimulation, the difference was statistically significant (P=0.041). Multivariate regression analysis showed that gender, age, body mass index, disease course and absolute power of the subjects had no statistical significance (all P>0.05). Conclusion After a single intervention of intermittent θ pulse stimulation, the absolute power of β-band of T7 and CP6 channels in the temporal region of type 2 diabetes mellitus patients with mild cognitive impairment was increased, and reduce the frequency ratio of the T7 channel. iTBS improves cognitive function by affecting cognitive processing in patients with type 2 diabetes with mild cognitive impairment. -
表 1 假刺激组与真刺激组T2DM合并MCI患者的一般临床资料比较(x ±s)
组别 例数 性别(男/女,例) 年龄(x±s, 岁) BMI(x±s) 病程(x±s,年) 受教育年限(x±s,年) 空腹血糖(x±s,mmol/L) MoCA量表(x±s) 日常生活能力量表(x±s) 老年抑郁量表(x±s) 总体衰退量表(二级/三级,例) 假刺激组 20 11/9 68.65±5.63 24.01±0.73 7.50±3.94 6.60±1.07 7.60±0.47 19.05±3.98 15.50±0.34 2.35±0.40 18/2 真刺激组 20 11/9 69.55±3.33 24.72±0.46 11.55±6.31 7.95±1.05 7.72±0.29 17.30±3.77 15.85±0.46 2.15±0.24 17/3 统计量 0.400a -0.615b -1.190b -2.434b -1.012b -0.717b 1.427b -0.368b -0.139b -0.472a P值 0.999 0.542 0.234 0.021 0.312 0.473 0.162 0.713 0.890 0.637 注:a为χ2值,b为t值。 表 2 真刺激组T2DM合并MCI患者刺激前后T7、CP6通道不同频段上频率的比较(x ±s,μV2)
项目 T7-1通道频率 T7-2通道频率 CP6-1通道频率 CP6-2通道频率 δ频段(1~4 Hz) 52.75±6.14 54.81±8.84 47.11±7.60 50.28±11.63 θ频段(4~8 Hz) 45.56±6.30 48.33±8.14 40.14±7.80 43.50±11.38 α频段(8~13 Hz) 45.00±7.45 48.26±8.29 39.51±8.97 42.66±11.76 β频段(13~30 Hz) 42.04±7.99 45.83±8.28a 36.25±8.11 39.84±11.10a 注: 与刺激前比较,aP < 0.05;-1指刺激前,-2指刺激后。 表 3 各因素及变量的赋值表
因素 变量 赋值说明 性别 X1 1=男性,2=女性 年龄(岁) X2 1=60~64,2=65~69,3=70~75 BMI X3 1=18.5~23.9,2=24.0~27.9,3=28.0~32.0 病程(年) X4 1=1~10,2=11~20,3=21~30 表 4 T7通道频率影响因素分析
变量 B SE B' t值 P值 X1 -2.359 3.272 -0.134 -0.721 0.476 X2 0.510 0.376 0.245 1.357 0.184 X3 0.462 0.650 0.141 0.711 0.482 X4 -0.130 -0.272 -0.081 -0.477 0.637 表 5 CP6通道频率影响因素分析
变量 B SE B' t值 P值 X1 -0.791 3.593 -0.041 -0.220 0.827 X2 0.452 0.412 0.201 1.097 0.281 X3 -0.113 0.714 -0.032 -0.158 0.875 X4 -0.232 0.299 -0.135 -0.777 0.443 表 6 真刺激组刺激前后T7与CP6通道频率比(x ±s)
项目 T7 CP6 刺激前 1.03±0.02 1.04±0.03 刺激后 1.02±0.02 1.04±0.02 t值 2.194 0.630 P值 0.041 0.536 -
[1] SAEDI E, GHEINI M R, FAIZ F, et al. Diabetes mellitus and cognitive impairments[J]. World J Diabetes, 2016, 7(17): 412-422. doi: 10.4239/wjd.v7.i17.412 [2] MUSAEUS C S, NIELSEN M S, HOGH P. Altered low-frequency EEG connectivity in mild cognitive impairment as a sign of clinical progression[J]. J Alzheimers Dis, 2019, 68(3): 947-960. doi: 10.3233/JAD-181081 [3] CHENG C P W, WONG C S M, LEE K K, et al. Effects of repetitive transcranial magnetic stimulation on improvement of cognition in elderly patients with cognitive impairment: A systematic review and meta-analysis[J]. Int J Geriatr Psychiatry, 2018, 33(1): e1-e13. doi: 10.1002/gps.4726 [4] BLUMBERGER D M, VILA-RODRIGUEZ F, THORPE K E, et al. Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): A randomised non-inferiority trial[J]. Lancet, 2018, 391(10131): 1683-1692. doi: 10.1016/S0140-6736(18)30295-2 [5] MAZZON G, DE DEA F, CATTARUZZA T, et al. Memorization test and resting state EEG components in mild and subjective cognitive impairment[J]. Curr Alzheimer Res, 2018, 15(9): 809-819. doi: 10.2174/1567205015666180427114520 [6] LAGE C, WILES K, SHERGILL S S, et al. A systematic review of the effects of low-frequency repetitive transcranial magnetic stimulation on cognition[J]. J Neural Transm (Vienna), 2016, 123(12): 1479-1490. doi: 10.1007/s00702-016-1592-8 [7] JIA W, WENG J, ZHU D, et al. Standards of medical care for type 2 diabetes in China 2019[J]. Diabetes Metab Res Rev, 2019, 35(6): e3158. http://d.wanfangdata.com.cn/periodical/3ece432d99cfb83e7d79b7561011a5e0 [8] PETERSEN R C, SMITH G E, WARING S C, et al. Mild cognitive impairment: Clinical characterization and outcome[J]. Arch Neurol, 1999, 56(3): 303-308. doi: 10.1001/archneur.56.3.303 [9] 吴伟, 朱小惠, 陶华英, 等. 非痴呆性血管性认知功能障碍脑梗死患者rTMS治疗的临床研究[J]. 中华全科医学, 2015, 13(3): 356-357, 514. https://www.cnki.com.cn/Article/CJFDTOTAL-SYQY201503006.htm [10] LOWE C J, MANOCCHIO F, SAFATI A B, et al. The effects of theta burst stimulation (TBS) targeting the prefrontal cortex on executive functioning: A systematic review and meta-analysis[J]. Neuropsychologia, 2018, 111: 344-359. doi: 10.1016/j.neuropsychologia.2018.02.004 [11] CHUNG S W, HOY K E, FITZGERALD P B. Theta-burst stimulation: A new form of TMS treatment for depression?[J]. Depress Anxiety, 2015, 32(3): 182-192. doi: 10.1002/da.22335 [12] TANG Y, YING C, WANG J, et al. Precise theta burst transcranial magnetic stimulation selectively reduced duration-related mismatch negativity[J]. Biol Psychol, 2018, 137: 125-132. doi: 10.1016/j.biopsycho.2018.08.001 [13] MIR-MOGHTADAEI A, CABALLERO R, FRIED P, et al. Concordance between beam F3 and MRI-neuronavigated target sites for repetitive transcranial magnetic stimulation of the left dorsolateral prefrontal cortex[J]. Brain Stimul, 2015, 8(5): 965-973. doi: 10.1016/j.brs.2015.05.008 [14] CHUNG S W, SULLIVAN C M, ROGASCH N C, et al. The effects of individualised intermittent theta burst stimulation in the prefrontal cortex: A TMS-EEG study[J]. Hum Brain Mapp, 2019, 40(2): 608-627. doi: 10.1002/hbm.24398 [15] BABILONI C, BARRY R J, BASAR E, et al. International Federation of Clinical Neurophysiology (IFCN) - EEG research workgroup: Recommendations on frequency and topographic analysis of resting state EEG rhythms. Part 1: Applications in clinical research studies[J]. Clin Neurophysiol, 2020, 131(1): 285-307. doi: 10.1016/j.clinph.2019.06.234 [16] 张慧, 张韶伟, 于德华, 等. 轻度认知功能障碍的药物治疗研究进展[J]. 中华全科医学, 2019, 17(9): 1571-1574, 1591. https://www.cnki.com.cn/Article/CJFDTOTAL-SYQY201909040.htm [17] SRIKANTH V, SINCLAIR A J, HILL-BRIGGS F, et al. Type 2 diabetes and cognitive dysfunction-towards effective management of both comorbidities[J]. Lancet Diabetes Endocrinol, 2020, 8(6): 535-545. doi: 10.1016/S2213-8587(20)30118-2 [18] COORAY G, NILSSON E, WAHLIN A, et al. Effects of intensified metabolic control on CNS function in type 2 diabetes[J]. Psychoneuroendocrinology, 2011, 36(1): 77-86. doi: 10.1016/j.psyneuen.2010.06.009 [19] OKAMURA H, JING H, TAKIGAWA M. EEG modification induced by repetitive transcranial magnetic stimulation[J]. J Clin Neurophysiol, 2001, 18(4): 318-325. doi: 10.1097/00004691-200107000-00003 [20] ROSSI S, PASQUALETTI P, ROSSINI P M, et al. Effects of repetitive transcranial magnetic stimulation on movement-related cortical activity in humans[J]. Cereb Cortex, 2000, 10(8): 802-808. doi: 10.1093/cercor/10.8.802 [21] MUSAEUS C S, NIELSEN M S, OSTERBYE N N, et al. Decreased parietal beta power as a sign of disease progression in patients with mild cognitive impairment[J]. J Alzheimers Dis, 2018, 65(2): 475-487. doi: 10.3233/JAD-180384 [22] ROH J H, PARK M H, KO D, et al. Region and frequency specific changes of spectral power in Alzheimer's disease and mild cognitive impairment[J]. Clin Neurophysiol, 2011, 122(11): 2169-2176. doi: 10.1016/j.clinph.2011.03.023 -





 下载:
下载: