The value of a novel automatic nucleic acid system in detecting Epstein-Barr virus for the rapid diagnosis of infectious mononucleosis in children
-
摘要:
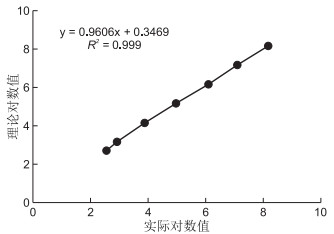
目的 对新型EB病毒(Epstein-Barr virus,EBV)全自动核酸定量检测系统进行性能验证,并探讨该系统在儿童传染性单核细胞增多症(IM)快速诊断中的价值。 方法 EBV全自动核酸定量检测系统包括核酸提取和实时荧光PCR(FQ-PCR)定量检测,分别采用PANA9600S全自动旋转式核酸工作站进行全血标本的核酸提取,ABI7500荧光PCR仪进行FQ-PCR扩增。然后验证EBV全自动核酸检测系统的性能指标,包括准确性、精密度、线性范围、检测限、防污染能力、交叉反应和抗干扰能力等。最后将EBV全自动核酸检测系统用于50例2018年11月—2019年6月温州医科大学附属第二医院的门诊及住院疑似IM患儿的核酸定量检测,并将EBV核酸检测结果与其他指标进行比较。 结果 EBV全自动核酸定量检测系统的准确性好,高、中、低水平的定量标准品均在定值范围内。检测精密度高,批内及批间精密度的CV值均<5%。该检测系统在1.0×103~1.00×108 copies/mL线性范围内,线性相关系数≥0.980,检测限为5.0×102 copies/mL。另外,该检测系统的防污染能力良好,无交叉反应,抗干扰能力强。对50例儿童疑似EBV感染者的外周血进行核酸检测,阳性检出率为80.0%,EBV定量值在5.45×102~1.46×108 copies/mL,明显高于血清EBV IgM抗体检测(P=0.031)和血常规异型淋巴细胞检测(P < 0.001)。 结论 EBV全自动核酸定量检测系统的各项性能指标良好,可满足临床检测要求,可较好地用于疑似儿童IM的快速诊断,值得临床应用推广。 Abstract:Objective To verify the performance of a novel automatic nucleic acid system in detecting Epstein-Barr virus (EBV) and evaluate its value in the rapid diagnosis of infectious mononucleosis (IM) in children. Methods The automatic EBV quantitative detection system involves nucleic acid extraction and quantitative detection via real-time fluorescence PCR. Whole blood samples were extracted using a PANA9600S automatic rotating nucleic acid workstation and amplified using an ABI7500 real-time fluorescence PCR. The main performance indexes of the EBV quantitative detection system, such as accuracy, precision, linear range, detection limit, antipollution ability, cross reaction and anti-interference ability, were evaluated. Finally, the detection system was used for nucleic acid quantitative detection of 50 children suspected with IM. The results of EBV nucleic acid detection were compared with those of other existing indicators. Results The EBV automatic quantitative detection system had good accuracy, and its high, medium and low levels of quantitative standards were within the range of fixed value. Its detection precision was high, and both CV values within and between batches were < 5%. The detection system was within the linear range of 1.0×103-1.0×108 copies/mL. The linear correlation coefficient was above 0.980, and the detection limit was 5.0×102 copies/mL. Moreover, the detection system had good antipollution ability, no cross reaction and had a strong anti-interference ability. EBV nucleic acid test was conducted on the peripheral blood of 50 children with suspected EBV infection. The positive detection rate was 80.0%, and the quantitative value of EBV ranged from 5.45×102 copies/mL to 1.46×108 copies/mL, which was significantly higher than the detection of serum EBV IgM antibody (P=0.031) and blood routine detection of abnormal lymphocyte ratio (P < 0.01). Conclusion The novel EBV automatic quantitative nucleic acid detection system described herein has good performance indexes and thus can meet the requirements of clinical detection. Therefore, it can be utilised for the rapid diagnosis of children with suspected IM. Thus, its clinical application must be promoted. -
表 1 批内和批间精密度验证
项目 x±s(log10,copies/mL) CV(%) Q水平 L水平 Q水平 L水平 批内精密度 6.86±0.11 4.14±0.09 1.65 2.10 批间精密度 6.81±0.13 4.15±0.12 1.86 2.79 表 2 临床样本的EBV定量检测结果(copies/mL)
EBV载量 例(%) EBV定值范围(中位数) <500 10(20.0) <5.00×102 102~103 17(34.0) 5.45×102~6.76×103(9.56×102) 104~105 17(34.0) 2.46×104~7.79×105(7.55×104) 106~107及以上 6(12.0) 1.15×106~1.46×108(2.56×107) 合计 50(100.0) 5.45×102~1.46×108(5.46×104) 表 3 EBV核酸检测结果与EB VCA IgM定性结果比较(例)
EBV检测结果 EB VCA IgM抗体检测结果 合计 阳性 阴性 阳性(≥500 copies/mL) 34 6 40 阴性(<500 copies/mL) 0 10 10 合计 34 16 50 表 4 EBV核酸检测结果与异型淋巴百分比比较(例)
EBV检测结果 异型淋巴百分比 合计 阳性(≥10%) 阴性(<10%) 阳性(≥500 copies/mL) 2 38 40 阴性(<500 copies/mL) 0 10 10 合计 2 48 50 -
[1] WINTER J R, JACKSON C, LEWIS J E, et al. Predictors of Epstein-Barr virus serostatus and implications for vaccine policy: A systematic review of the literature[J]. J Glob Health, 2020, 10(1): 010404. doi: 10.7189/jogh.10.010404 [2] 郭睿, 李奇玉. 儿童传染性单核细胞增多症的诊断与治疗进展[J]. 中国临床实用医学, 2019, 10(2): 78-80. https://www.cnki.com.cn/Article/CJFDTOTAL-SYYZ200809004.htm [3] 夏玉雪, 方峻. EB病毒与传染性单核细胞增多症的研究进展[J]. 临床内科杂志, 2019, 36(6): 371-374. doi: 10.3969/j.issn.1001-9057.2019.06.004 [4] 邓坤仪, 彭建明, 范汉恭, 等. 三种实验室检测方法在儿童传染性单核细胞增多症的诊断应用[J]. 分子诊断与治疗杂志, 2016, 8(1): 37-41. doi: 10.3969/j.issn.1674-6929.2016.01.007 [5] 谢正德, 刘春艳, 艾军红. EB病毒感染实验室诊断及临床应用专家共识[J]. 中华实验和临床病毒学杂志, 2018, 32(1): 2-8. doi: 10.3760/cma.j.issn.1003-9279.2018.01.001 [6] 江杨华, 宋然, 刘巧突, 等. 郴州地区350例健康儿童EBV感染情况分析[J]. 实用预防医学, 2020, 27(6): 689-692. https://www.cnki.com.cn/Article/CJFDTOTAL-SYYY202006014.htm [7] NANBO A, KACHI K, YOSHIYAMA H, et al. Epstein-Barr virus exploits host endocytic machinery for cell-to-cell viral transmission rather than a virological synapse[J]. J Gen Virol, 2016, 97(11): 2989-3006. doi: 10.1099/jgv.0.000605 [8] 谢正德, 张蕊, 俞蕙. 儿童主要非肿瘤性EB病毒感染相关疾病的诊断和治疗原则建议[J]. 中华儿科杂志, 2016, 54(8): 563-568. doi: 10.3760/cma.j.issn.0578-1310.2016.08.002 [9] CLSI. CLSI documents EP15-A2 User verification of performance for precision and trueness; approved guideline-second edition[S]. Wayne, PA: CLSI, 2008. [10] CLSI. CLSI documents EP6-A Evaluation of the linearity of Quantitative Measurement Procedures: A Statistical Approach. Approved guideline[S]. Wayne, PA: CLSI, 2003. [11] 中华人民共和国国家卫生和计划生育委员会. GB/T 20468-2006临床实验室定量测定室内质量控制指南[S]. 北京: 中国标准出版社, 2016. [12] SMATTI M K, AL-SADEQ D W, ALI N H, et al. Epstein-Barr virus epidemiology, serology, and genetic variability of LMP-1 oncogene among healthy population: An update[J]. Front Oncol, 2018, 8: 211. doi: 10.3389/fonc.2018.00211 [13] PICH D, MROZEK-GORSKA P, BOUVET M, et al. First days in the life of naive human B lymphocytes infected with Epstein-Barr virus[J]. MBio, 2019, 10(5): e01723-e01719. http://www.ncbi.nlm.nih.gov/pubmed/31530670 [14] MVNZ C. Latency and lytic replication in Epstein-Barr virus-associated oncogenesis[J]. Nat Rev Microbiol, 2019, 17(11): 691-700. doi: 10.1038/s41579-019-0249-7 [15] 郑炜琨, 何时军, 李素华. EB病毒核酸测定在儿童EB病毒感染中的应用价值[J]. 中国妇幼保健, 2017, 32(15): 3552-3554. https://www.cnki.com.cn/Article/CJFDTOTAL-ZFYB201715053.htm [16] 许青, 于新发. 血浆EB病毒-DNA检测在鼻咽癌中的应用[J]. 海南医学, 2020, 31(1): 114-117. https://www.cnki.com.cn/Article/CJFDTOTAL-HAIN202001033.htm [17] 朱艳, 周灵玲, 蓝俊伟, 等. 人巨细胞病毒DNA检测在小儿人巨细胞病毒感染诊断中的应用[J]. 中华全科医学, 2020, 18(3): 460-462, 466. https://www.cnki.com.cn/Article/CJFDTOTAL-SYQY202003033.htm [18] 沈妙娜, 黄燕, 黄倩雯, 等. 儿童感染EB病毒后核酸拷贝分析[J]. 国际医药卫生导报, 2019(17): 2911-2912. doi: 10.3760/cma.j.issn.1007-1245.2019.17.019 [19] BYRNE A, BUSH R, JOHNS F, et al. Limited utility of serology and heterophile test in the early diagnosis of Epstein-Barr virus mononucleosis in a child after renal transplantation[J]. Medicines(Basel), 2020, 7(4): 21. http://www.researchgate.net/publication/340865990_Limited_Utility_of_Serology_and_Heterophile_Test_in_the_Early_Diagnosis_of_Epstein-Barr_Virus_Mononucleosis_in_a_Child_after_Renal_Transplantation/download [20] 陈静, 史利欢, 谢昕. 血清抗体联合DNA检测在儿童EB病毒感染中的诊断效能分析[J]. 中国民康医学, 2019, 31(9): 116-118. https://www.cnki.com.cn/Article/CJFDTOTAL-ZMYX201909052.htm [21] ABRAS A, BALLART C, LLOVET T, et al. Introducing automation to the molecular diagnosis of Trypanosoma cruzi infection: a comparative study of sample treatments, DNA extraction methods and real-time PCR assays[J]. PLoS One, 2018, 13(4): e0195738. http://www.ncbi.nlm.nih.gov/pubmed/29664973 -





 下载:
下载: