Application of nomogram in the prognostic evaluation of patients after intrahepatic cholangiocarcinoma resection
-
摘要:
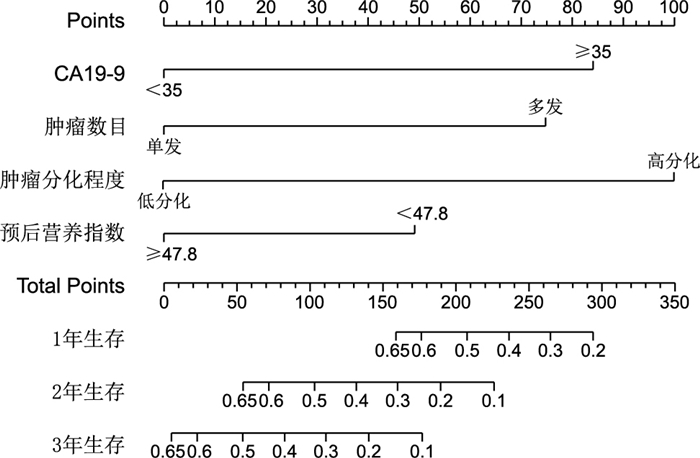
目的 分析肝内胆管癌根治性切除术后患者的生存因素并建立列线图,评估列线图能否有效预测个体的生存时间。 方法 回顾性分析蚌埠医学院第一附属医院2013年1月—2019年12月80例接受根治性肝切除术肝内胆管癌患者临床病理资料,采用Cox比例风险回归模型分析影响患者生存的独立危险因素,运用R语言建立根治性切除术后肝内胆管癌患者生存列线图,使用一致性指数C和校准曲线验证列线图性能,并使用ROC曲线下面积比较列线图和TNM分期对术后患者生存的预测效果。 结果 Cox多因素回归分析结果糖链蛋白抗原19-9(CA19-9)、肿瘤数目、肿瘤分化程度、预后营养指数为肝内胆管癌术后患者生存时间的独立影响因素,并构建列线图。列线图一致性指数C为0.692,校准曲线图接近对角线,表明列线图具有较好的区分度和准确性,列线图时间依赖性ROC曲线预测肝内胆管癌术后患者1、2、3年生存率的AUC分别为0.783、0.711、0.726。列线图ROC曲线下面积为0.741,大于TNM分期ROC曲线下面积0.509。 结论 由CA19-9、肿瘤数目、肿瘤分化程度、预后营养指数构建的列线图比TNM分期能更有效地预测肝内胆管癌术后患者生存时间,并具有估算个体的生存概率能力。 Abstract:Objective To analyse the survival factors of patients with intrahepatic cholangiocarcinoma after radical resection and establish a nomogram to evaluate whether the nomogram can effectively predict individual survival time. Methods The clinicopathological data of 80 patients with intrahepatic cholangiocarcinoma who underwent radical hepatectomy in the First Affiliated Hospital of Bengbu Medical College from January 2013 to December 2019 were retrospectively analysed. Cox regression model was used to analyse the independent risk factors affecting the survival of patients, and R language was used to establish survival nomogram of patients with intrahepatic cholangiocarcinoma after radical resection. The consistency index and calibration curve were used to verify the performance of the nomogram, and the area under the ROC curve (AUC) was used to compare the predictive effect of the nomogram and TNM staging on postoperative patient survival. Results Multivariate Cox analysis showed that CA19-9, tumour number, tumour differentiation and prognostic nutritional index were independent risk factors affecting the survival time of patients with intrahepatic cholangiocarcinoma after surgery, and a nomogram was constructed. The nomogram consistency index C was 0.692, and the calibration curve was close to the diagonal line, indicating that the nomogram had good differentiation and accuracy. The AUC values of the nomogram time-dependent ROC curve for predicting the 1-, 2- and 3-year survival rates of patients with intrahepatic cholangiocarcinoma after surgery were 0.783, 0.711 and 0.726, respectively. The AUC of the nomogram was 0.741, which was greater than that of TNM staging (0.509). Conclusion The nomogram constructed from CA19-9, tumour number, tumour differentiation and prognostic nutritional index is more effective than TNM staging in predicting the survival time of postoperative patients with intrahepatic cholangiocarcinoma and has the ability to estimate individual survival probability. -
Key words:
- Intrahepatic cholangiocarcinoma /
- Nomogram /
- Prognosis
-
表 1 80例肝内胆管癌患者一般资料
Table 1. General information of 80 patients with intrahepatic bile duct cancer
项目 类别 例数 百分比(%) 项目 类别 例数 百分比(%) 性别 男性 50 62.5 肝段切除 <3个 32 40.0 女性 30 37.5 ≥3个 48 60.0 年龄(岁) ≤60 32 40.0 淋巴结清扫 有 31 38.8 >60 48 60.0 无 49 61.2 NLR ≥1.2 46 57.5 糖尿病 有 27 33.8 <1.2 34 42.5 无 53 66.2 结石史 有 28 35.0 肿瘤直径 ≤5 38 47.5 无 52 65.0 (cm) >5 42 52.5 肝硬化 有 22 27.5 无 58 72.5 表 2 80例肝内胆管癌患者总生存时间单因素分析
Table 2. Univariate analysis of overall survival time in 80 patients with intrahepatic cholangiocarcinoma
项目 例数 OS(月) HR值 95% CI P值 CA19-9(U/mL) 2.683 1.576~4.574 <0.001 ≥35 50 15.0 <35 30 32.0 PNI 0.458 0.285~0.784 0.004 ≥47.8 55 22.0 <47.8 25 10.0 肿瘤数目(个) 1.788 1.054~2.957 0.020 单发 51 24.0 多发 29 15.0 淋巴结转移 1.712 1.013~2.886 0.047 无 58 23.0 有 22 11.0 血管侵犯 2.889 1.632~5.117 <0.001 无 25 37.0 有 55 15.0 肿瘤分化程度 1.766 1.072~3.041 0.028 低分化 56 22.0 高分化 24 10.0 乙肝病毒感染 0.491 0.274~0.908 0.023 无 58 16.0 有 22 34.0 TNM分期 2.087 1.186~3.684 0.011 Ⅰ期 21 26.0 Ⅱ~Ⅲ期 59 15.0 注:TNM分期AJCC第8版肝内胆管癌TNM分期。 表 3 80例肝内胆管癌患者OS的多因素分析
Table 3. Multifactorial analysis of OS in 80 patients with intrahepatic cholangiocarcinoma
项目 B SE Wald χ2 P值 OR值 95% CI PNI -0.596 0.298 3.990 0.046 0.551 0.307~0.989 TNM 0.389 0.357 1.191 0.275 1.476 0.734~2.970 CA19-9 0.846 0.375 5.084 0.024 2.331 1.117~4.865 肿瘤数目 0.892 0.376 5.626 0.018 2.440 1.168~5.098 淋巴结转移 0.433 0.298 2.108 0.147 1.542 0.859~2.768 血管侵犯 -0.327 0.513 0.406 0.524 0.721 0.264~1.972 乙肝病毒感染 -0.620 0.413 2.258 0.133 0.538 0.240~1.208 肿瘤分化程度 0.755 0.356 4.516 0.034 2.129 1.060~4.273 -
[1] MACIAS R I R, MONTE M J, SERRANO M A, et al. Impact of aging on primary liver cancer: Epidemiology, pathogenesis and therapeutics[J]. Aging (Albany NY), 2021, 13(19): 23416-23434. [2] IOFFE D, PHULL P, DOTAN E. Optimal management of patients with advanced or metastatic cholangiocarcinoma: An evidence-based review[J]. Cancer Manag Res, 2021, 13: 8085-8098. doi: 10.2147/CMAR.S276104 [3] WATANABE Y, MATSUYAMA Y, IZUMI N, et al. Effect of surgical margin width after R0 resection for intrahepatic cholangiocarcinoma: A nationwide survey of the Liver Cancer Study Group of Japan[J]. Surgery, 2020, 167: 793-802. doi: 10.1016/j.surg.2019.12.009 [4] 李传涛, 周迟, 崔培元, 等. 术前NLR和PLR与肝门胆管癌根治术后临床病理特征及T分期的关系[J]. 中华全科医学, 2021, 19(7): 1095-1098. doi: 10.16766/j.cnki.issn.1674-4152.001993LI C T, ZHOU C, CUI P, et al. Preoperative NLR and PLR and radical resection of hilar cholangiocarcinoma Relationship between clinicopathological features and T staging[J]. Chinese Journal of General Practice, 2021, 19(7): 1095-1098. doi: 10.16766/j.cnki.issn.1674-4152.001993 [5] BAGANTE F, MERATH K, SQUIRES M H, et al. The limitations of standard clinicopathologic features to accurately risk-stratify prognosis after resection of intrahepatic cholangiocarcinoma[J]. J Gastrointest Surg, 2018, 22(3): 477-485. doi: 10.1007/s11605-018-3682-4 [6] MATSUDA T, UMEDA Y, MATSUDA T, et al. Preoperative prognostic nutritional index predicts postoperative infectious complications and oncological outcomes after hepatectomy in intrahepatic cholangiocarcinoma[J]. BMC Cancer, 2021, 21(1): 1-12. doi: 10.1186/s12885-020-07763-8 [7] FU J, CHEN Q J J, YU Y Y, et al. Impact of portal hypertension on short-and long-term outcomes after liver resection for intrahepatic cholangiocarcinoma: A propensity score matching analysis[J]. Cancer Med, 2021, 10(20): 6985-6997. doi: 10.1002/cam4.4222 [8] SIM J H, KIM S H, JUN I G, et al. The association between prognostic nutritional index (PNI) and intraoperative transfusion in patients undergoing hepatectomy for hepatocellular carcinoma: A Retrospective cohort study[J]. Cancers (Basel), 2021, 13(11): 2508. doi: 10.3390/cancers13112508 [9] ITOH S, TSUJITA E, FUKUZAWA K, et al. Prognostic significance of preoperative PNI and CA19-9 for pancreatic ductal adenocarcinoma: A multi-institutional retrospective study[J]. Pancreatology, 2021, 21(7): 1356-1363. doi: 10.1016/j.pan.2021.08.003 [10] MORO A, MEHTA R, SAHARA K, et al. The impact of preoperative CA19-9 and CEA on outcomes of patients with intrahepatic cholangiocarcinoma[J]. Ann Surg Oncol, 2020, 27(8): 2888-2901. doi: 10.1245/s10434-020-08350-8 [11] ZHANG X Y, ZHOU Y J, WU Z R, et al. Double-negative α-fetoprotein and carbohydrate antigen 19-9 predict a good prognosis in intrahepatic cholangiocarcinoma: A propensity score matching analysis[J]. Clin Transl Gastroenterol, 2021, 12(11): e00425. DOI: 10.14309/ctg.0000000000000425. [12] BUETTNER S, TEN CATE D W G, BAGANTE F, et al. Survival after resection of multiple tumor foci of intrahepatic cholangiocarcinoma[J]. J Gastrointest Surg, 2019, 23(11): 2239-2246. doi: 10.1007/s11605-019-04184-2 [13] OKUBO S, MITSUNAGA S, KATO Y, et al. The prognostic impact of differentiation at the invasive front of biliary tract cancer[J]. J Surg Oncol, 2018, 117: 1278-1287. doi: 10.1002/jso.24946 [14] KEMI N, ESKURI M, IKÄLÄINEN J, et al. Tumor budding and prognosis in gastric adenocarcinoma[J]. Am J Surg Pathol, 2019, 43: 229-234. doi: 10.1097/PAS.0000000000001181 [15] CHRISTOFI T, BARITAKI S, FALZONE L, et al. Current perspectives in cancer immunotherapy[J]. Cancers(Basel), 2019, 11(10): 1472. [16] SCHARPING N E, RIVADENEIRA D B, MENK A V, et al. Mitochondrial stress induced by continuous stimulation under hypoxia rapidly drives T cell exhaustion[J]. Nat Immunol, 2021, 22(2): 205-215. doi: 10.1038/s41590-020-00834-9 [17] KEAVER L, HOULIHAN C, O'CALLAGHAN N, et al. Evidence-based nutrition guidelines for cancer survivors in Europe: A call for action[J]. Eur J Clin Nutr, 2021, 29: 1-8. -





 下载:
下载: