The influence of liraglutide on insulin secretion function of E23K mutation of Kir6.2 gene in β cells
-
摘要:
目的 Kir6.2编码基因多态与糖尿病、胰岛素抵抗相关,本研究旨在探讨高糖环境下,利拉鲁肽对Kir6.2基因E23K位点突变的胰岛β细胞胰岛素分泌功能的影响及机制。 方法 以NIT-1细胞为研究对象,构建Kir6.2基因过表达野生型和E23K突变型NIT-1细胞,并用利拉鲁肽在高糖环境下进行24 h干预。采用流式细胞仪检测NIT-1细胞凋亡率、细胞膜电位及钙离子浓度,Western blotting和免疫荧光检测细胞内胰岛素含量,ELISA检测培养液中胰岛素含量,并以此间接研究胰岛素的分泌情况。 结果 NIT-1细胞最适高糖培养浓度为60 mmol/L,在此浓度下胰岛素合成量最高,利拉鲁肽体外干预浓度选择10 mmol/L。过表达野生型Kir6.2基因NIT-1细胞的胰岛素分泌高于Kir6.2基因E23K突变型NIT-1细胞。过表达野生型Kir6.2基因NIT-1细胞在高糖环境中细胞膜电位进一步降低,细胞内Ca2+含量增加,而Kir6.2基因E23K突变型NIT-1细胞与单纯高糖培养相比无显著改变。利拉鲁肽在高糖环境中可有效减少野生型和突变型NIT-1细胞的凋亡,降低膜电位,增加细胞内Ca2+含量,促进胰岛素分泌。另外利拉鲁肽对维持突变型NIT-1细胞存活作用更显著。 结论 在高糖环境中,利拉鲁肽能改善Kir6.2基因E23K位点多态型胰岛β细胞的胰岛素分泌功能,该研究为利拉鲁肽的临床应用提供基础研究证据。 Abstract:Objective Polymorphism of Kir6.2 is associated with diabetes and insulin resistance. This study aims to explore whether liraglutide can improve the insulin secretion function and mechanism of the E23K mutation of Kir6.2 gene of β cell of islet under a high glucose condition. Methods Taking NIT-1 cells as the object, Kir6.2 gene-overexpressed wild-type and E23K mutant NIT-1 cells were constructed, and liraglutide was adopted to intervene in a high-glucose environment for 24 hours. Flow cytometry was used to detect the NIT-1 cell apoptosis rate, the cell membrane potential and the calcium ion concentration. Western blotting and immunofluorescence assay were conducted to detect insulin contents in cells. ELISA was utilized to detect insulin contents in nutrient fluid, based on which insulin secretion was indirectly studied. Results NIT-1 cells had an optimal high glucose culture concentration of 60 mmol/L, with the highest amount of insulin synthesis, whereas 10 mmol/L was selected for liraglutide in vitro. The insulin secretion of Kir6.2 gene-overexpressed wild-type NIT-1 cells was higher than that of Kir6.2 gene E23K mutant NIT-1 cells. The Kir6.2 gene-overexpressed wild-type NIT-1 cells presented less membrane potential and higher Ca2+ concentration in cells in a high-glucose environment, while Kir6.2 gene E23 mutant NIT-1 cells made no significant changes compared with those cultured under high glucose concentration. Liraglutide could effectively reduce apoptosis of wild-type and mutant NIT-1 cells, decrease membrane potential, increase Ca2+ concentration in the cells, and promote insulin secretion in a high-glucose environment. In addition, liraglutide was of greater significance to the survival of mutant NIT-1 cells. Conclusion In a high-glucose environment, liraglutide can improve the insulin secretion function of polymorphic of E23K site Kir6.2 gene islet β cell. This study provides basic evidence for the clinical application of liraglutide. -
Key words:
- Liraglutide /
- Kir6.2 /
- E23K mutation /
- Islet βcell /
- Insulin
-
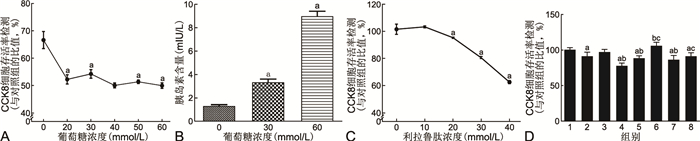
图 1 最适高糖培养浓度及利拉鲁肽体外干预浓度
注:A为不同浓度D-葡萄糖引起NIT-1细胞活性的改变;B为ELISA检测不同浓度D-葡萄糖介导的胰岛素分泌量;C为不同浓度利拉鲁肽干预引起的NIT-1细胞活性的改变;D为各实验组细胞活性的比较;与对照组比较,aP < 0.05;与高糖组比较,bP < 0.05;与未经利拉鲁肽预处理的对应实验组比较,cP < 0.05。D中1为对照组,2为高糖组,3为高糖+载体阴性对照组,4为高糖+Kir6.2野生型组,5为高糖+Kir6.2突变型组,6为高糖+载体阴性对照+利拉鲁肽组,7为高糖+Kir6.2野生型+利拉鲁肽组,8为高糖+Kir6.2突变型+利拉鲁肽组。
Figure 1. The optimal high glucose culture concentration and in vitro intervention concentration of liraglutide
图 2 利拉鲁肽对各组NIT-1细胞膜电位的影响
注:高糖以及利拉鲁肽对细胞膜电位的作用。与对照组比较,aP < 0.05;与高糖组比较,bP < 0.05;与未经利拉鲁肽预处理的对应实验组比较,cP < 0.05。1为对照组,2为高糖组,3为高糖+载体阴性对照组,4为高糖+Kir6.2野生型组,5为高糖+Kir6.2突变型组,6为高糖+载体阴性对照+利拉鲁肽组,7为高糖+Kir6.2野生型+利拉鲁肽组,8为高糖+Kir6.2突变型+利拉鲁肽组。
Figure 2. Effect of liraglutide on the membrane potential of NIT-1 cells in each group
图 3 利拉鲁肽对各组NIT-1细胞钙离子内流的影响
注:流式细胞仪检测胞内钙离子含量。与对照组比较,aP < 0.05;与高糖组比较,bP < 0.05;与未经利拉鲁肽预处理的对应实验组比较,cP < 0.05。1为对照组,2为高糖组,3为高糖+载体阴性对照组,4为高糖+Kir6.2野生型组,5为高糖+Kir6.2突变型组,6为高糖+载体阴性对照+利拉鲁肽组,7为高糖+Kir6.2野生型+利拉鲁肽组,8为高糖+Kir6.2突变型+利拉鲁肽组。
Figure 3. Effect of liraglutide on calcium influx in NIT-1 cells of each group
表 1 各组NIT-1细胞凋亡率的比较(x±s,%)
Table 1. Comparison of apoptosis rates of NIT-1 cells in each group(x±s, %)
组别 样本量 细胞凋亡率 对照组 6 8.51±0.37 高糖组 6 26.24±1.17a 高糖+载体阴性对照组 6 26.95±0.72a 高糖+Kir6.2野生型 6 42.11±0.54a 高糖+Kir6.2突变型 6 26.19±0.50ab 高糖+载体阴性对照+利拉鲁肽 6 11.81±0.36abc 高糖+Kir6.2野生型+利拉鲁肽 6 24.25±0.32abc 高糖+Kir6.2突变型+利拉鲁肽 6 27.30±0.96abc 注:与对照组比较,aP < 0.05;与高糖组比较,bP < 0.05;与未经利拉鲁肽预处理的对应实验组比较,cP < 0.05。 -
[1] LIU J D, YU D Q, XU M Y, et al. β-Cell function is associated with osteosarcopenia in middle-aged and older nonobese patients with type 2 diabetes: a cross-sectional study[J]. Open Med (Wars), 2021, 16(1): 1583-1590. doi: 10.1515/med-2021-0376 [2] YANG Y, KIM J W, PARK H S, et al. Pancreatic stellate cells in the islets as a novel target to preserve the pancreatic βcell mass and function[J]. J Diabetes Investig, 2020, 11(2): 268-280. doi: 10.1111/jdi.13202 [3] WANG D D, CHEN X, YANG Y, et al. Association of Kir6.2 gene rs5219 variation with type 2 diabetes: a meta-analysis of 21, 464 individuals[J]. Prim Care Diabetes, 2018, 12(4): 345-353. doi: 10.1016/j.pcd.2018.03.004 [4] FADWA E O, MOHAMED E. Asteriscus graveolens exhibits antihypertensive activity through activation of vascular KATP channels activation in rats[J]. Endocr Metab Immune Disord Drug Targets, 2020, 20(5): 736-744. doi: 10.2174/1871530319666191016100851 [5] MENG G L, ZHAO S, XIE L P, et al. Protein S-sulfhydration by hydrogen sulfide in cardiovascular system[J]. Br J Pharmacol, 2018, 175(8): 1146-1156. doi: 10.1111/bph.13825 [6] RIZVI S, RAZA S T, MAHDI F, et al. Genetic polymorphisms in KCNJ11(E23K, rs5219) and SDF-1β(G801A, rs1801157) genes are associated with the risk of type 2 diabetes mellitus[J]. Br J Biomed Sci, 2018, 75(3): 139-144. doi: 10.1080/09674845.2018.1473939 [7] MAKHZOOM O, KABALAN Y, FAIZEH A L Q. Association of KCNJ11 rs5219 gene polymorphism with type 2 diabetes mellitus in a population of Syria: a case-control study[J]. BMC Med Genet, 2019, 20(1): 1-6. doi: 10.1186/s12881-018-0738-y [8] REN Y X, ZHU W F, SHI J K, et al. Association between KCNJ11 E23K polymorphism and the risk of type 2 diabetes mellitus: a global meta-analysis[J]. J Diabetes Complications, 2022, 36(5): 108170. DOI: 10.1016/j.jdiacomp.2022.108170. [9] MUFTIN N Q, JUBAIR S. KCNJ11 polymorphism is associated with type 2 diabetes mellitus in Iraqi patients[J]. Gene Rep, 2019, 17: 100480. DOI: 10.1016/j.genrep.2019.100480. [10] 孙露, 张力, 杨晓晖. 常用口服降糖药分类及临床合理使用(续完)[J]. 中华全科医学, 2017, 15(12): 2008-2009. http://www.zhqkyx.net/article/id/8182cc19-30dc-42dc-b497-bb0e005f0035SUN L, ZHANG L, YANG X H. Classification and clinical rational use of commonly used oral hypoglycemic drugs (continued)[J]. Chinese Journal of General Practice, 2017(12): 2008-2009. http://www.zhqkyx.net/article/id/8182cc19-30dc-42dc-b497-bb0e005f0035 [11] LIU H Z, LUO B, CHEN X, et al. Preserved pharmacokinetics and pharmacodynamics of insulin degludec and liraglutide when administered as insulin degludec/liraglutide in a Chinese population[J]. J Diabetes Investig, 2022, 13(4): 652-656. doi: 10.1111/jdi.13716 [12] MIKHAIL N. Cardiovascular effects of liraglutide[J]. Curr Hypertens Rev, 2019, 15(1): 64-69. doi: 10.2174/1573402114666180507152620 [13] ZENG Z W, HUANG S Y, SUN T. Pharmacogenomic studies of current antidiabetic agents and potential new drug targets for precision medicine of diabetes[J]. Diabetes Ther, 2020, 11(11): 2521-2538. doi: 10.1007/s13300-020-00922-x [14] 王宏伟, 杨岚, 江思瑜, 等. 利拉鲁肽对2型糖尿病大鼠胰岛β细胞凋亡的调控机制[J]. 中国老年医学杂志, 2019, 39(3): 646-650. https://www.cnki.com.cn/Article/CJFDTOTAL-ZLXZ201903048.htmWANG W H, YANG L, JIANG S Y, et al. Apoptosis mechanism of liraglutide against rat islet beta cells in type 2 dia-betes mellitus rats[J]. Chinese Journal of Gerontology, 2019, 34(3): 646-650. https://www.cnki.com.cn/Article/CJFDTOTAL-ZLXZ201903048.htm [15] RABBONE I, BARBETTI F, GENTILELLA R, et al. Insulin therapy in neonatal diabetes mellitus: a review of the literature[J]. Diabetes Res Clin Pract, 2017, 129: 126-135. doi: 10.1016/j.diabres.2017.04.007 -





 下载:
下载: