Comparison of the efficacy of surgical resection and radiofrequency ablation after chemotherapy for resectable colorectal liver metastases
-
摘要:
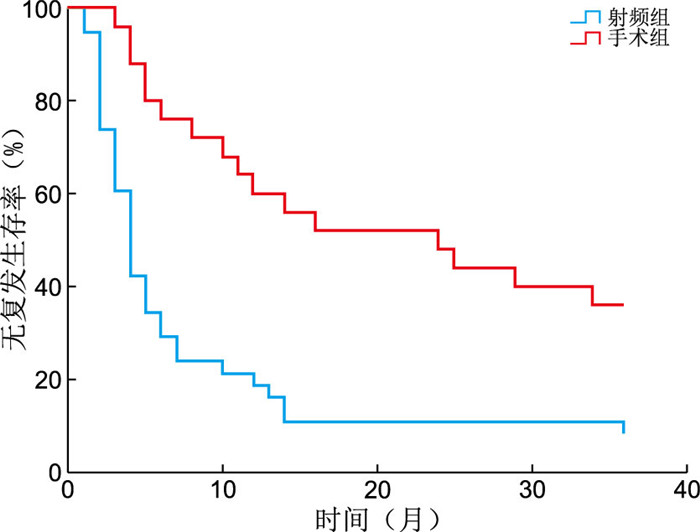
目的 临床上对于肠癌肝转移患者往往会采取全身化疗序贯局部治疗的治疗模式,但对于化疗后肝转移灶局部治疗方式的选择尚未达成共识。本文拟通过评价手术切除和射频消融2种方法对此类患者的疗效来为临床医生提供参考依据。 方法 回顾性分析2017年1月1日—12月31日期间在浙江省肿瘤医院接受肝脏局部治疗(手术或射频消融术)且术前经过全身化疗的63例结直肠癌肝转移患者的临床资料。63例患者根据局部治疗方式的不同分为手术组和射频组,手术组25例患者接受肝切除术,射频组38例患者接受射频消融术。通过分析手术组和射频组患者的局部无复发生存时间及1年、2年、3年无复发生存率以比较手术切除和射频消融2种方法对化疗后结直肠癌肝转移灶的疗效。 结果 2组患者肿瘤数目及肿瘤大小比较差异无统计学意义。经过最长36个月的随访,手术组中有64.0%(16/25)的患者出现了肝脏局部复发,射频组中92.1%(35/38)的患者出现了肝脏局部复发。手术组中位无复发时间(24.0个月,95% CI:6.0~42.0个月)与射频组(4.0个月,95% CI:3.1~4.9个月)比较差异有统计学意义(P < 0.05)。手术组患者的1年、2年、3年肝脏无复发生存率分别为64.0%、52.0%、36.0%,射频组患者为21.1%、10.5%、10.5%,2组比较差异均有统计学意义(均P < 0.05)。 结论 对于经过全身化疗后肝脏病灶可切除的结直肠癌肝转移患者,手术切除可能给患者带来更低的局部复发率和更长的局部无复发生存时间。 Abstract:Objective Systemic chemotherapy followed by local treatment is recommended for patients with colorectal liver metastases (CRLMs). However, there is no consensus on the choice of local treatment for CRLMs after chemotherapy. The research aims to evaluate the efficacy of surgical resection and radiofrequency ablation (RFA) in these patients to provide some information for clinicians. Methods The clinical data of 63 patients with colorectal liver metastases who accepted chemotherapy and underwent local treatment (surgical resection or RFA) at Zhejiang Cancer hospital from January 1, 2017 to December 31, 2017 were retrospectively analyzed. All patients were divided into two groups according to the different methods of local treatment: hepatectomy group and RFA group. The 25 patients in hepatectomy group accepted hepatectomy and the 38 patients in RFA group accepted RFA. The efficacy of surgical resection and RFA in the treatment of colorectal liver metastases (CRLMs) was compared by analyzing local Recurrence-Free Survival time and Recurrence-Free Survival rates of 1 year, 2 years and 3 years. Results There was no significant difference in the number and maximum size of CRLMs between the hepatectomy and RFA groups. Patients were followed for 36 months. Local liver recurrences occurred in 16 patients in the hepatectomy group (64.0%) and 35 patients in the RFA group (92.1%). The median RFS time in the hepatectomy group (24.0 months, 95% CI: 6.0-42.0 months) was significant longer than the RFA group (4.0 months, 95% CI: 3.1-4.9 months, P < 0.05). The 1-year, 2-years and 3-years RFS rates were 64.0%, 52.0% and 36.0% in the hepatectomy group and 21.1%, 10.5%, 10.5% in the RFA group. There were significant differences between the two groups (all P < 0.05). Conclusion For some patients with resectable CRLMs who have accepted chemotherapy, surgical resection may result in a lower rate of local recurrence and longer RFS. -
Key words:
- Colorectal cancer /
- Liver metastases /
- Hepatectomy /
- Radiofrequency ablation
-
表 1 2组结直肠癌肝转移患者一般资料比较
Table 1. Comparison of general characteristics between two groups of patients with CRLMs
组别 例数 年龄(x±s,岁) 性别(女性/男性,例) 手术组 25 55.76±14.39 8/17 射频组 38 60.26±11.81 10/28 统计量 1.357a 0.239b P值 0.180 0.625 注:a为t值,b为χ2值。 表 2 2组结直肠癌肝转移患者肝转移灶特征比较[M(P25, P75)]
Table 2. Comparison of hepatic metastatic lesion characteristics in two groups of CRLMs [M(P25, P75)]
组别 例数 肿瘤数目(个) 肿瘤大小(cm) 手术组 25 2.00(1.00, 3.00) 2.50(1.40, 4.45) 射频组 38 1.00(1.00, 2.00) 2.45(2.00, 3.33) Z值 -0.749 -0.127 P值 0.454 0.899 -
[1] SUNG H, FERLAY J, SIEGEL R L, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2021, 71(3): 209-249. doi: 10.3322/caac.21660 [2] TAKAHASHI H, BERBER E. Role of thermal ablation in the management of colorectal liver metastasis[J]. Hepatobiliary Surg Nutr, 2020, 9(1): 49-58. doi: 10.21037/hbsn.2019.06.08 [3] 鲁立峰, 盛勤松, 邹德胜, 等. 结直肠癌切除联合射频消融对同时性结直肠癌肝转移临床指标、安全性及预后的影响[J]. 中华全科医学, 2018, 16(5): 736-739. doi: 10.16766/j.cnki.issn.1674-4152.000200LU L F, SHENG Q S, ZOU D S, et al. Effect of resectional therapy combined with radiofrequency ablation on clinical indicators, safety and prognosis of patients with colorectal liver metastasis[J]. Chinese Journal of General Practice, 2018, 16(5): 736-739. doi: 10.16766/j.cnki.issn.1674-4152.000200 [4] GIANNIS D, SIDERIS G, KAKOS C D, et al. The role of liver transplantation for colorectal liver metastases: a systematic review and pooled analysis[J]. Transplant Rev (Orlando), 2020, 34(4): 100570. DOI: 10.1016/j.trre.2020.100570. [5] NIEUWENHUIZEN S, DIJKSTRA M, PUIJK R S, et al. Thermal ablation versus stereotactic ablative body radiotherapy to treat unresectable colorectal liver metastases: a comparative analysis from the prospective amsterdam CORE registry[J]. Cancers (Basel), 2021, 13(17): 4303. DOI: 10.3390/cancers13174303. [6] NIEUWENHUIZEN S, DIJKSTRA M, PUIJK R S, et al. Microwave ablation, radiofrequency ablation, irreversible electroporation, and stereotactic ablative body radiotherapy for intermediate size (3-5 cm) unresectable colorectal liver metastases: a systematic review and meta-analysis[J]. Curr Oncol Rep, 2022, 24(6): 793-808. doi: 10.1007/s11912-022-01248-6 [7] ELFRINK A K E, NIEUWENHUIZEN S, VAN DEN TOL M P, et al. Hospital variation in combined liver resection and thermal ablation for colorectal liver metastases and impact on short-term postoperative outcomes: a nationwide population-based study[J]. HPB (Oxford), 2021, 23(6): 827-839. doi: 10.1016/j.hpb.2020.10.003 [8] ADAM R, KITANO Y. Multidisciplinary approach of liver metastases from colorectal cancer[J]. Ann Gastroenterol Surg, 2019, 3(1): 50-56. doi: 10.1002/ags3.12227 [9] SILVEIRA JUNIOR S, TUSTUMI F, MAGALHAES D P, et al. The impact of multivisceral liver resection on short- and long-term outcomes of patients with colorectal liver metastasis: a systematic review and meta-analysis[J]. Clinics (Sao Paulo), 2022, 77: 100099. DOI: 10.1016/j.clinsp.2022.100099. [10] ENGSTRAND J, NILSSON H, STROMBERG C, et al. Colorectal cancer liver metastases: a population-based study on incidence, management and survival[J]. BMC Cancer, 2018, 18(1): 78. doi: 10.1186/s12885-017-3925-x [11] YUAN Z J, WENG S S, YE C Y, et al. CSCO guidelines for colorectal cancer version 2022: updates and discussions[J]. Chin J Cancer Res, 2022, 34(2): 67-70. doi: 10.21147/j.issn.1000-9604.2022.02.01 [12] SIBINGA MULDER B G, VISSER K, FESHTALI S, et al. Gadoxetic acid-enhanced magnetic resonance imaging significantly influences the clinical course in patients with colorectal liver metastases[J]. BMC Med Imaging, 2018, 18(1): 44. doi: 10.1186/s12880-018-0289-x [13] MALEUX G, IZAMIS M L, WERBROUCK C, et al. Characterization of liver metastases during catheter-directed liver interventions: a comparison between dual phase cone-beam computed tomography and conventional contrast-enhanced computed tomography[J]. J Belg Soc Radiol, 2020, 104(1): 41. doi: 10.5334/jbsr.2052 [14] LIU J L, BAO D, XU Z L, et al. Clinical value of contrast-enhanced computed tomography (CECT) combined with contrast-enhanced ultrasound (CEUS) for characterization and diagnosis of small nodular lesions in liver[J]. Pak J Med Sci, 2021, 37(7): 1843-1848. [15] CHEN J Y, DAI H Y, LI C Y, et al. Improved sensitivity and positive predictive value of contrast-enhanced intraoperative ultrasound in colorectal cancer liver metastasis: a systematic review and meta-analysis[J]. J Gastrointest Oncol, 2022, 13(1): 221-230. doi: 10.21037/jgo-21-881 -





 下载:
下载: