Clinical study on the therapeutic effect and prognostic value of peripheral blood inflammatory indexes in the treatment of advanced gastric cancer with immune checkpoint inhibitors
-
摘要:
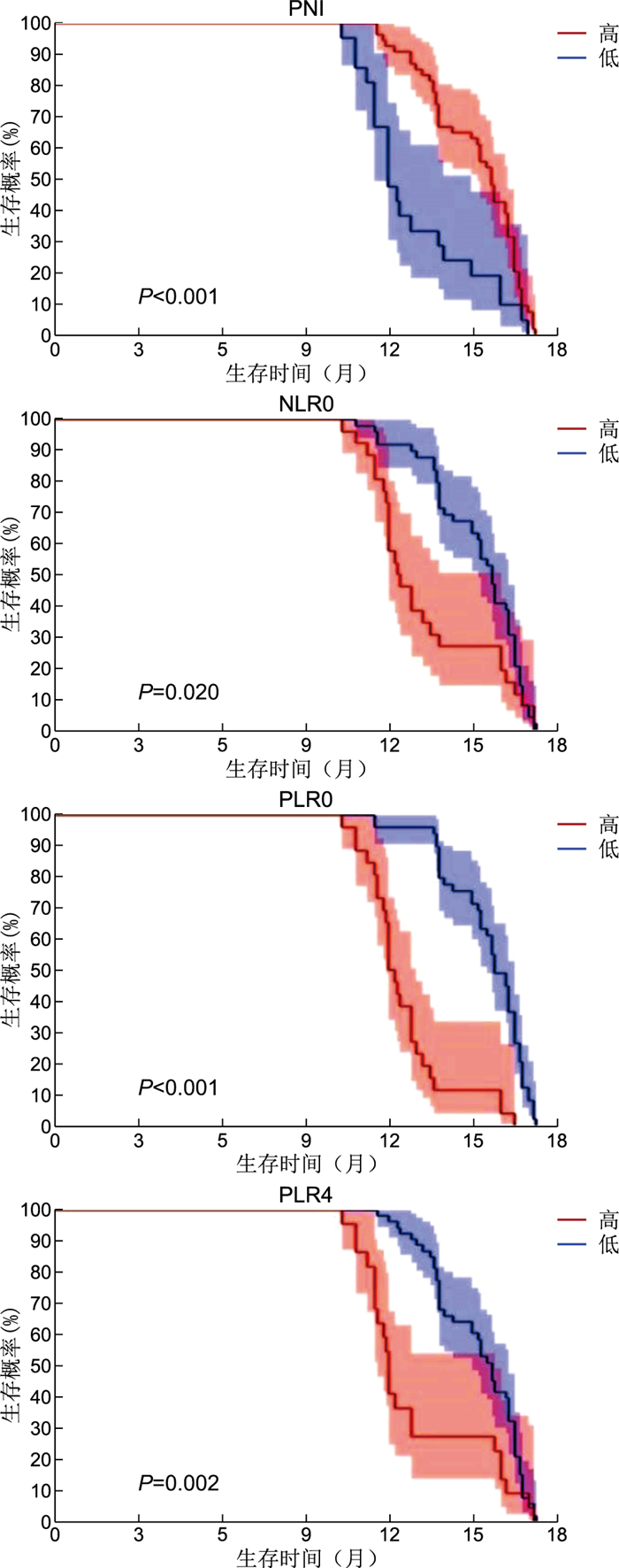
目的 探究预后营养指数(PNI)、中性粒细胞与淋巴细胞比值(NLR)、血小板与淋巴细胞比值(PLR)对接受免疫检查点抑制剂(ICIs)治疗的晚期胃癌(AGC)疗效及预后价值评估。 方法 回顾性分析新乡医学院第一附属医院2019年6月—2022年1月接受ICIs治疗75例AGC患者的临床资料。应用ROC曲线计算PNI、NLR、PLR最佳截断值。绘制Kaplan-Meier曲线并运用Cox比例风险模型预测影响AGC总生存期(OS)的独立危险因素。 结果 PNI、NLR0、PLR0、PLR4的最佳截断值为36.00、3.45、186.65、138.23。高PNI组和低PLR0组客观缓解率(ORR)更高;高PNI组、低NLR0组、低PLR0组和低PLR4组疾病控制率(DCR)更高,差异均有统计学意义(P<0.05)。单因素分析显示,PNI、NLR0、PLR0、PLR4、BMI、分期均与OS相关。多因素分析显示,分期(HR=2.040,95% CI:1.120~3.730,P=0.020)及PLR0(HR=3.539,95% CI:1.717~7.296,P<0.001)是OS的独立影响因素。 结论 PNI、NLR0、PLR0、PLR4对接受ICIs治疗的AGC疗效及预后具有临床指导价值。 Abstract:Objective To explore the efficacy and prognostic value of prognostic nutrition index (PNI), neutrophil to lymphocyte ratio (NLR), platelet to lymphocyte ratio (PLR) in advanced gastric cancer (AGC) treated with immune checkpoint inhibitors (ICIs). Methods A retrospective analysis was conducted on the clinical data of 75 AGC patients who received ICIs treatment at the First Affiliated Hospital of Xinxiang Medical College from June 2019 to January 2022. The ROC curve was used to calculate the optimal cutoff values for PNI, NLR, and PLR. Kaplan-Meier curves was plotted, and cox regression analysis was used to predict the independent risk factors affecting overall survival (OS) in AGC. Results The best cutoff values for PNI, NLR0, PLR0, and PLR4 were 36.00, 3.45, 186.65, and 138.23, respectively. The objective response rate (ORR) of high PNI group and low PLR0 group were higher. The disease control rate (DCR) of high PNI group, low NLR0 group, low PLR0 group and low PLR4 group were higher, and the differences were statistically significant (P<0.05). Univariate analysis showed that PNI, NLR0, PLR0, PLR4, BMI and stage were correlated with OS. Multivariate analysis showed that PLR0 was an independent risk factor for OS (HR=3.539, 95% CI: 1.717-7.296, P<0.001) and PFS (HR=4.556, 95% CI: 1.955-10.617, P<0.001) except stage. Conclusion Detection of PNI, NLR0, PLR0, and PLR4 has clinical guiding value for the efficacy and prognosis of AGC after ICIs treatment. -
表 1 不同水平PNI、NLR0、PLR0及PLR4的AGC患者治疗后ORR和DCR比较[例(%)]
Table 1. Comparison of ORR and DCR after treatment in AGC patients with different levels of PNI, NLR0, PLR0 and PLR4 [cases (%)]
组别 例数 ORR DCR 低PNI组(PNI≤36.00) 22 1(4.76) 5(22.73) 高PNI组(PNI>36.00) 53 15(28.30) 49(92.45) χ2值 3.908 37.491 P值 0.048 <0.001 低NLR0组(NLR≤3.45) 49 13(26.53) 45(91.84) 高NLR0组(NLR>3.45) 26 3(11.54) 9(34.62) χ2值 2.275 27.589 P值 0.131 <0.001 低PLR0组(PLR≤186.65) 49 15(30.61) 44(89.79) 高PLR0组(PLR>186.65) 26 1(3.85) 9(34.62) χ2值 7.252 24.952 P值 0.007 <0.001 低PLR4组(PLR≤138.23) 53 13(24.53) 48(90.56) 高PLR4组(PLR>138.23) 22 3(13.64) 6(27.27) χ2值 0.546 30.893 P值 0.460 <0.001 表 2 影响晚期胃癌患者OS的单因素和多因素分析
Table 2. Univariate and multivariate analysis of OS in patients with advanced gastric cancer
变量 单因素分析 多因素分析 HR值 95% CI P值 HR值 95% CI P值 高PNI(参照:低PNI) 0.424 0.253~0.711 <0.001 0.537 0.276~1.044 0.067 高NLR0(参照:低NLR0) 1.710 1.050~2.780 0.020 0.727 0.349~1.511 0.393 高PLR0(参照:低PLR0) 5.702 3.299~9.855 <0.001 3.539 1.717~7.296 <0.001 高PLR4(参照:低PLR4) 2.087 1.258~3.463 0.002 1.457 0.735~2.888 0.280 高BMI(参照:低BMI) 0.485 0.302~0.780 0.003 0.910 0.510~1.630 0.750 分期Ⅳ期(参照:Ⅲ期) 2.296 1.360~3.876 0.002 2.040 1.120~3.730 0.020 -
[1] SUNG H, FERLAY J, SIEGEL R L, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2021, 71(3): 209-249. doi: 10.3322/caac.21660 [2] SMYTH E C, NILSSON M, GRABSCH H I, et al. Gastric cancer[J]. Lancet, 2020, 396(10251): 635-648. doi: 10.1016/S0140-6736(20)31288-5 [3] WANG H, GUO W H, HU Y F, et al. Superiority of the 8th edition of the TNM staging system for predicting overall survival in gastric cancer: comparative analysis of the 7th and 8th editions in a monoinstitutional cohort[J]. Mol Clin Oncol, 2018, 9(4): 423-431. [4] JOSHI S S, BADGWELL B D. Current treatment and recent progress in gastric cancer[J]. CA Cancer J Clin, 2021, 71(3): 264-279. doi: 10.3322/caac.21657 [5] JANJIGIAN Y Y, SHITARA K, MOEHLER M, et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial[J]. Lancet, 2021, 398(10294): 27-40. doi: 10.1016/S0140-6736(21)00797-2 [6] KANG Y K, CHEN L T, RYU M H, et al. Nivolumab plus chemotherapy versus placebo plus chemotherapy in patients with HER2-negative, untreated, unresectable advanced or recurrent gastric or gastro-oesophageal junction cancer (ATTRACTION-4): a randomised, multicentre, double-blind, placebo-controlled, phase 3 trial[J]. Lancet Oncol, 2022, 23(2): 234-247. doi: 10.1016/S1470-2045(21)00692-6 [7] SHITARA K, VANCUTSEM E, BANG Y J, et al. Efficacy and safety of pembrolizumab or pembrolizumab plus chemotherapy vs chemotherapy alone for patients with first-line, advanced gastric cancer: the KEYNOTE-062 phase 3 randomized clinical trial[J]. JAMA Oncol, 2020, 6(10): 1571-1580. doi: 10.1001/jamaoncol.2020.3370 [8] LINHARES P, FERREIRA A, VAZ R. The importance of the neutrophil-to-lymphocyte ratio in the prognosis of glioma and its subtypes[J]. CNS Neurosci Ther, 2020, 26(3): 394-395. doi: 10.1111/cns.13270 [9] TAKECHI H, FUJIKUNI N, TANABE K, et al. Using the preoperative prognostic nutritional index as a predictive factor for non-cancer-related death in post-curative resection gastric cancer patients: a retrospective cohort study[J]. BMC Gastroenterol, 2020, 20(1): 256. doi: 10.1186/s12876-020-01402-z [10] DU S T, FANG Z H, YE L, et al. Pretreatment neutrophil-to-lym-phocyte ratio predicts the benefit of gastric cancer patients with systemic therapy[J]. Aging(Albany NY), 2021, 13(13): 17638-17654. [11] WU X B, HAN R K, ZHONG Y P, et al. Post treatment NLR is a predictor of response to immune checkpoint inhibitor therapy in patients with esophageal squamous cell carcinoma[J]. Cancer Cell Int, 2021, 21(1): 356. doi: 10.1186/s12935-021-02072-x [12] WANG F H, ZHANG X T, LI Y F, et al. The Chinese Society of Clinical Oncology (CSCO): clinical guidelines for the diagnosis and treatment of gastric cancer, 2021[J]. Cancer Commun(Lond), 2021, 41(8): 747-795. [13] 马静, 胡博, 羊樟福, 等. 抗PD-1/PD-L1抗体单药及联合治疗晚期胆道癌相关不良反应管理进展[J]. 中国临床医学, 2022, 29(6): 1022-1029. https://www.cnki.com.cn/Article/CJFDTOTAL-LCYX202206023.htmMA J, HU B, YANG Z F, et al. The adverse events management of anti-PD-1/PD-L1 antibody monotherapy and combination therapy in the treatment of advanced biliary tract cancer[J]. Chinese Journal of Clinical Medicine, 2022, 29(6): 1022-1029. https://www.cnki.com.cn/Article/CJFDTOTAL-LCYX202206023.htm [14] ZHOU Y X, ZHANG Y Q, GUO G F, et al. Nivolumab plus ipilimumab versus pembrolizumab as chemotherapy-free, first-line treatment for PD-L1-positive non-small cell lung cancer[J]. Clin Transl Med, 2020, 10(1): 107-115. doi: 10.1002/ctm2.14 [15] BODOR J N, BOUMBER Y, BORGHAEI H. Biomarkers for immune checkpoint inhibition in non-small cell lung cancer (NSCLC)[J]. Cancer, 2020, 126(2): 260-270. doi: 10.1002/cncr.32468 [16] DENK D, GRETEN F R. Inflammation: the incubator of the tumor microenvironment[J]. Trends Cancer, 2022, 8(11): 901-914. doi: 10.1016/j.trecan.2022.07.002 [17] SAHU A, KOSE K, KRAEHENBUEHL L, et al. In vivo tumor immune microenvironment phenotypes correlate with inflammation and vasculature to predict immunotherapy response[J]. Nat Commun, 2022, 13(1): 5312. doi: 10.1038/s41467-022-32738-7 [18] SHI T, ZHANG Y P, WANG Y, et al. DKK1 promotes tumor immune evasion and impedes anti-PD-1 treatment by inducing immunosuppressive macrophages in gastric cancer[J]. Cancer Immunol Res, 2022, 10(12): 1506-1524. doi: 10.1158/2326-6066.CIR-22-0218 [19] YAMAMOTO T, KAWADA K, OBAMA K. Inflammation-related biomarkers for the prediction of prognosis in colorectal cancer patients[J]. Int J Mol Sci, 2021, 22(15): 8002. DOI: 10.3390/ijms22158002. [20] 杨丽萍, 吴爱林, 周燕, 等. 放疗前NLR和PLR对局部晚期鼻咽癌患者预后的影响[J]. 中华全科医学, 2022, 20(9): 1458-1461, 1534. doi: 10.16766/j.cnki.issn.1674-4152.002622YANG L P, WU A L, ZHOU Y, et al. Effect of NLR and PLR from pre-radiotherapy on prognosis of locally advanced nasopharyngeal carcinoma patients[J]. Chinese Journal of General Practice, 2022, 20(9): 1458-1461. doi: 10.16766/j.cnki.issn.1674-4152.002622 [21] JIANG Y, XU D, SONG H, et al. Inflammation and nutrition-based biomarkers in the prognosis of oesophageal cancer: a systematic review and meta-analysis[J]. BMJ Open, 2021, 11(9): e048324. DOI: 10.1136/bmjopen-2020-048324. [22] 把明勤, 陈柳, 朱小琼, 等. NRS2002和PNI营养筛查方法在胃癌患者预后中的预测价值[J]. 中华全科医学, 2023, 21(7): 1121-1124. doi: 10.16766/j.cnki.issn.1674-4152.003064BA M Q, CHEN L, ZHU X Q, et al. Predictive value of NRS2002 and PNI nutritional screening methods in the prognosis of patients with gastric cancer[J]. Chinese Journal of General Practice, 2023, 21(7): 1121-1124. doi: 10.16766/j.cnki.issn.1674-4152.003064 [23] FENG J F, WANG L, YANG X, et al. Prediction of pathologic complete response prediction in patients with locally advanced esophageal squamous cell carcinoma treated with neoadjuvant immunochemotherapy: a real-world study[J]. Biomol Biomed, 2023, 23(1): 153-160. [24] LIU J J, LI S, ZHANG S, et al. Systemic immune-inflammation index, neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio can predict clinical outcomes in patients with metastatic non-small-cell lung cancer treated with nivolumab[J]. J Clin Lab Anal, 2019, 33(8): e22964. DOI: 10.1002/jcla.22964. -





 下载:
下载: